Different correlation patterns of cholinergic and GABAergic interneurons with striatal projection neurons
- PMID: 24027501
- PMCID: PMC3760072
- DOI: 10.3389/fnsys.2013.00047
Different correlation patterns of cholinergic and GABAergic interneurons with striatal projection neurons
Abstract
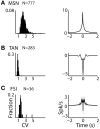
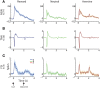
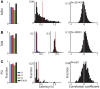
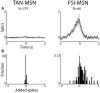
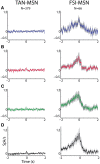
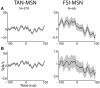
The striatum is populated by a single projection neuron group, the medium spiny neurons (MSNs), and several groups of interneurons. Two of the electrophysiologically well-characterized striatal interneuron groups are the tonically active neurons (TANs), which are presumably cholinergic interneurons, and the fast spiking interneurons (FSIs), presumably parvalbumin (PV) expressing GABAergic interneurons. To better understand striatal processing it is thus crucial to define the functional relationship between MSNs and these interneurons in the awake and behaving animal. We used multiple electrodes and standard physiological methods to simultaneously record MSN spiking activity and the activity of TANs or FSIs from monkeys engaged in a classical conditioning paradigm. All three cell populations were highly responsive to the behavioral task. However, they displayed different average response profiles and a different degree of response synchronization (signal correlation). TANs displayed the most transient and synchronized response, MSNs the most diverse and sustained response and FSIs were in between on both parameters. We did not find evidence for direct monosynaptic connectivity between the MSNs and either the TANs or the FSIs. However, while the cross correlation histograms of TAN to MSN pairs were flat, those of FSI to MSN displayed positive asymmetrical broad peaks. The FSI-MSN correlogram profile implies that the spikes of MSNs follow those of FSIs and both are driven by a common, most likely cortical, input. Thus, the two populations of striatal interneurons are probably driven by different afferents and play complementary functional roles in the physiology of the striatal microcircuit.
Keywords: crosscorrealtion; interneurons; physiology; spikes; striatum.
Figures


References
-
- Abeles M. (1982b). Local Cortical Circuits. Berlin; Heidelberg; New York: Springer-Verlag
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

