Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease
- PMID: 24027553
- PMCID: PMC3759803
- DOI: 10.3389/fneur.2013.00122
Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease
Abstract
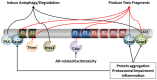
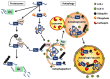
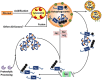
One of the defining pathological features of Alzheimer disease (AD) is the intraneuronal accumulation of tau. The tau that forms these accumulations is altered both posttranslationally and conformationally, and there is now significant evidence that soluble forms of these modified tau species are the toxic entities rather than the insoluble neurofibrillary tangles. However there is still noteworthy debate concerning which specific pathological forms of tau are the contributors to neuronal dysfunction and death in AD. Given that increases in aberrant forms of tau play a role in the neurodegeneration process in AD, there is growing interest in understanding the degradative pathways that remove tau from the cell, and the selectivity of these different pathways for various forms of tau. Indeed, one can speculate that deficits in a pathway that selectively removes certain pathological forms of tau could play a pivotal role in AD. In this review we will discuss the different proteolytic and degradative machineries that may be involved in removing tau from the cell. How deficits in these different degradative pathways may contribute to abnormal accumulation of tau in AD will also be considered. In addition, the issue of the selective targeting of specific tau species to a given degradative pathway for clearance from the cell will be addressed.
Keywords: autophagy; degradation; proteasome; proteolysis; tau.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

