Regulation of ras exchange factors and cellular localization of ras activation by lipid messengers in T cells
- PMID: 24027568
- PMCID: PMC3762125
- DOI: 10.3389/fimmu.2013.00239
Regulation of ras exchange factors and cellular localization of ras activation by lipid messengers in T cells
Abstract
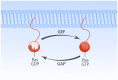
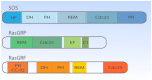
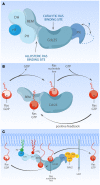
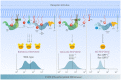
The Ras-MAPK signaling pathway is highly conserved throughout evolution and is activated downstream of a wide range of receptor stimuli. Ras guanine nucleotide exchange factors (RasGEFs) catalyze GTP loading of Ras and play a pivotal role in regulating receptor-ligand induced Ras activity. In T cells, three families of functionally important RasGEFs are expressed: RasGRF, RasGRP, and Son of Sevenless (SOS)-family GEFs. Early on it was recognized that Ras activation is critical for T cell development and that the RasGEFs play an important role herein. More recent work has revealed that nuances in Ras activation appear to significantly impact T cell development and selection. These nuances include distinct biochemical patterns of analog versus digital Ras activation, differences in cellular localization of Ras activation, and intricate interplays between the RasGEFs during distinct T cell developmental stages as revealed by various new mouse models. In many instances, the exact nature of these nuances in Ras activation or how these may result from fine-tuning of the RasGEFs is not understood. One large group of biomolecules critically involved in the control of RasGEFs functions are lipid second messengers. Multiple, yet distinct lipid products are generated following T cell receptor (TCR) stimulation and bind to different domains in the RasGRP and SOS RasGEFs to facilitate the activation of the membrane-anchored Ras GTPases. In this review we highlight how different lipid-based elements are generated by various enzymes downstream of the TCR and other receptors and how these dynamic and interrelated lipid products may fine-tune Ras activation by RasGEFs in developing T cells.
Keywords: LAT; P38; Ras; RasGRP; SOS; T cell; lipids; signaling.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

