Reducing levels of toxic RNA with small molecules
- PMID: 24028068
- PMCID: PMC4108295
- DOI: 10.1021/cb400431f
Reducing levels of toxic RNA with small molecules
Abstract
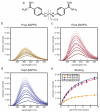
Myotonic dystrophy (DM) is one of the most common forms of muscular dystrophy. DM is an autosomal dominant disease caused by a toxic gain of function RNA. The toxic RNA is produced from expanded noncoding CTG/CCTG repeats, and these CUG/CCUG repeats sequester the Muscleblind-like (MBNL) family of RNA binding proteins. The MBNL proteins are regulators of alternative splicing, and their sequestration has been linked with mis-splicing events in DM. A previously reported screen for small molecules found that pentamidine was able to improve splicing defects associated with DM. Biochemical experiments and cell and mouse model studies of the disease indicate that pentamidine and related compounds may work through binding the CTG*CAG repeat DNA to inhibit transcription. Analysis of a series of methylene linker analogues of pentamidine revealed that heptamidine reverses splicing defects and rescues myotonia in a DM1 mouse model.
Figures
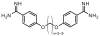





Similar articles
-
Pentamidine reverses the splicing defects associated with myotonic dystrophy.Proc Natl Acad Sci U S A. 2009 Nov 3;106(44):18551-6. doi: 10.1073/pnas.0903234106. Epub 2009 Oct 12. Proc Natl Acad Sci U S A. 2009. PMID: 19822739 Free PMC article.
-
Furamidine Rescues Myotonic Dystrophy Type I Associated Mis-Splicing through Multiple Mechanisms.ACS Chem Biol. 2018 Sep 21;13(9):2708-2718. doi: 10.1021/acschembio.8b00646. Epub 2018 Aug 27. ACS Chem Biol. 2018. PMID: 30118588 Free PMC article.
-
(CCUG)n RNA toxicity in a Drosophila model of myotonic dystrophy type 2 (DM2) activates apoptosis.Dis Model Mech. 2017 Aug 1;10(8):993-1003. doi: 10.1242/dmm.026179. Epub 2017 Jun 16. Dis Model Mech. 2017. PMID: 28623239 Free PMC article.
-
RNA-mediated therapies in myotonic dystrophy.Drug Discov Today. 2018 Dec;23(12):2013-2022. doi: 10.1016/j.drudis.2018.08.004. Epub 2018 Aug 4. Drug Discov Today. 2018. PMID: 30086404 Review.
-
Therapeutic Approaches for Dominant Muscle Diseases: Highlight on Myotonic Dystrophy.Curr Gene Ther. 2015;15(4):329-37. doi: 10.2174/1566523215666150630120537. Curr Gene Ther. 2015. PMID: 26122101 Review.
Cited by
-
Biological Efficacy and Toxicity of Diamidines in Myotonic Dystrophy Type 1 Models.J Med Chem. 2015 Aug 13;58(15):5770-80. doi: 10.1021/acs.jmedchem.5b00356. Epub 2015 Jul 21. J Med Chem. 2015. PMID: 26103061 Free PMC article.
-
Molecular characterization of myotonic dystrophy fibroblast cell lines for use in small molecule screening.iScience. 2022 Apr 4;25(5):104198. doi: 10.1016/j.isci.2022.104198. eCollection 2022 May 20. iScience. 2022. PMID: 35479399 Free PMC article.
-
The Dimeric Form of 1,3-Diaminoisoquinoline Derivative Rescued the Mis-splicing of Atp2a1 and Clcn1 Genes in Myotonic Dystrophy Type 1 Mouse Model.Chemistry. 2020 Nov 11;26(63):14305-14309. doi: 10.1002/chem.202001572. Epub 2020 Oct 6. Chemistry. 2020. PMID: 32449537 Free PMC article.
-
Design of novel small molecule base-pair recognizers of toxic CUG RNA transcripts characteristics of DM1.Comput Struct Biotechnol J. 2020 Dec 6;19:51-61. doi: 10.1016/j.csbj.2020.11.053. eCollection 2021. Comput Struct Biotechnol J. 2020. PMID: 33363709 Free PMC article.
-
The evolution and application of RNA-focused small molecule libraries.RSC Chem Biol. 2025 Feb 13;6(4):510-527. doi: 10.1039/d4cb00272e. eCollection 2025 Apr 2. RSC Chem Biol. 2025. PMID: 39957993 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

