Peanut Allergen Threshold Study (PATS): validation of eliciting doses using a novel single-dose challenge protocol
- PMID: 24028324
- PMCID: PMC3850217
- DOI: 10.1186/1710-1492-9-35
Peanut Allergen Threshold Study (PATS): validation of eliciting doses using a novel single-dose challenge protocol
Abstract
Background: The eliciting dose (ED) for a peanut allergic reaction in 5% of the peanut allergic population, the ED05, is 1.5 mg of peanut protein. This ED05 was derived from oral food challenges (OFC) that use graded, incremental doses administered at fixed time intervals. Individual patients' threshold doses were used to generate population dose-distribution curves using probability distributions from which the ED05 was then determined. It is important to clinically validate that this dose is predictive of the allergenic response in a further unselected group of peanut-allergic individuals.
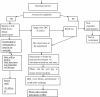
Methods/aims: This is a multi-centre study involving three national level referral and teaching centres. (Cork University Hospital, Ireland, Royal Children's Hospital Melbourne, Australia and Massachusetts General Hospital, Boston, U.S.A.) The study is now in process and will continue to run until all centres have recruited 125 participates in each respective centre.A total of 375 participants, aged 1-18 years will be recruited during routine Allergy appointments in the centres. The aim is to assess the precision of the predicted ED05 using a single dose (6 mg peanut = 1.5 mg of peanut protein) in the form of a cookie. Validated Food Allergy related Quality of Life Questionnaires-(FAQLQ) will be self-administered prior to OFC and 1 month after challenge to assess the impact of a single dose OFC on FAQL. Serological and cell based in vitro studies will be performed.
Conclusion: The validation of the ED05 threshold for allergic reactions in peanut allergic subjects has potential value for public health measures. The single dose OFC, based upon the statistical dose-distribution analysis of past challenge trials, promises an efficient approach to identify the most highly sensitive patients within any given food-allergic population.
Figures
References
-
- Allen KJ, Remington BC, Baumert JL, Crevel R, Houben GF, Brooks-Taylor S, Clinical challenge data for development of allergen management thresholds for precautionary labeling of foods- VITAL 2.0. J Allergy Clin Immun. 2013. p. . In press. - PubMed
-
- Taylor SL, Moneret-Vautrin DA, Crevel RWR, Sheffield D, Morisset M, Dumont P. et al.Threshold dose for peanut: risk characterization based upon diagnostic oral challenge of a series of 286 peanut-allergic individuals. Food Chem Toxicol. 2010;48(3):814–819. doi: 10.1016/j.fct.2009.12.013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous