Factors predicting late recurrence for estrogen receptor-positive breast cancer
- PMID: 24029245
- PMCID: PMC3787911
- DOI: 10.1093/jnci/djt244
Factors predicting late recurrence for estrogen receptor-positive breast cancer
Abstract
Background: Adjuvant endocrine therapy beyond 5 years reduces recurrence in patients with estrogen receptor-positive breast cancer. We have previously shown that immunohistochemical markers (IHC4) and two gene expression profile tests (recurrence score [RS] and PAM50 risk of recurrence [ROR]) are associated with time to distant recurrence, and we have now assessed the value of each of these scores and routine clinical variables for predicting outcome, specifically in years 5 to 10.
Methods: We used univariate and multivariable proportional hazards models to determine the prognostic value of all variables and scores (IHC4, RS, ROR) for distant recurrence, separately in years 0 to 5 and specifically for years 5 to 10 for all patients. All statistical tests were two-sided.
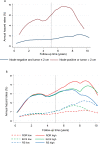
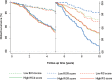
Results: Nodal status and tumor size were at least as strong in years 5 to 10 as in years 0 to 5 (nodal status, years 5-10: χ² = 21.72 vs years 0-5: χ² = 11.08, both P < .001; tumor size, years 5-10: χ² = 10.52 vs years 0-5: χ² = 10.82, both P = .001). Ki67 and the overall IHC4 score were the only statistically significant biomarkers related to distant recurrence univariablely in the 5 to 10 year period (χ² = 8.67, χ² = 13.22, respectively). The ROR score was the strongest molecular prognostic factor in the late follow-up period (χ² = 16.29; P < .001), whereas IHC4 (χ² = 7.41) and RS (χ² = 5.55) were only weakly prognostic in this period. Similar results were seen for all subgroups and for all recurrences.
Conclusions: None of the IHC4 markers provided statistically significant prognostic information in years 5 to 10, except for nodal status and tumor size. ROR gave the strongest prognostic information in years 5 to 10. These results may help select patients who could benefit most from hormonal therapy beyond 5 years of treatment.
Figures

References
-
- Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746 - PubMed
-
- Tamoxifen for early breast cancer: an overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114): 1451–1467 - PubMed
-
- Tamoxifen for early breast cancer. Cochrane Database Syst Rev. 2001;CD000486 DOI:10.1002/14651858 - PubMed
-
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–1141 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

