Libman-Sacks endocarditis and embolic cerebrovascular disease
- PMID: 24029368
- PMCID: PMC3941465
- DOI: 10.1016/j.jcmg.2013.04.012
Libman-Sacks endocarditis and embolic cerebrovascular disease
Abstract
Objectives: The aim of this study was to determine whether Libman-Sacks endocarditis is a pathogenic factor for cerebrovascular disease (CVD) in systemic lupus erythematosus (SLE).
Background: A cardioembolic pathogenesis of SLE CVD manifested as: 1) neuropsychiatric systemic lupus erythematosus (NPSLE), including stroke and transient ischemic attacks (TIA); 2) neurocognitive dysfunction; and 3) magnetic resonance imaging of focal brain lesions has not been established.
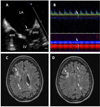
Methods: A 6-year study of 30 patients with acute NPSLE (27 women, 38 ± 12 years of age), 46 age- and sex-matched SLE controls without NPSLE (42 women, 36 ± 12 years of age), and 26 age- and sex-matched healthy controls (22 women, 34 ± 11 years of age) who underwent clinical and laboratory evaluations, transesophageal echocardiography, carotid duplex ultrasound, transcranial Doppler ultrasound, neurocognitive testing, and brain magnetic resonance imaging/magnetic resonance angiography. Patients with NPSLE were re-evaluated after 4.5 months of therapy. All patients were followed clinically for a median of 52 months.
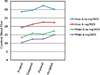
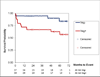
Results: Libman-Sacks vegetations (87%), cerebromicroembolism (27% with 2.5 times more events per hour), neurocognitive dysfunction (60%), and cerebral infarcts (47%) were more common in NPSLE than in SLE (28%, 20%, 33%, and 0%) and healthy controls (8%, 0%, 4%, and 0%, respectively) (all p ≤ 0.009). Patients with vegetations had 3 times more cerebromicroemboli per hour, lower cerebral blood flow, more strokes/TIA and overall NPSLE events, neurocognitive dysfunction, cerebral infarcts, and brain lesion load than those without (all p ≤ 0.01). Libman-Sacks vegetations were independent risk factors of NPSLE (odds ratio [OR]: 13.4; p < 0.001), neurocognitive dysfunction (OR: 8.0; p = 0.01), brain lesions (OR: 5.6; p = 0.004), and all 3 outcomes combined (OR: 7.5; p < 0.001). Follow-up re-evaluations in 18 of 23 (78%) surviving patients with NPSLE demonstrated improvement of vegetations, microembolism, brain perfusion, neurocognitive dysfunction, and lesion load (all p ≤ 0.04). Finally, patients with vegetations had reduced event-free survival time to stroke/TIA, cognitive disability, or death (p = 0.007).
Conclusions: The presence of Libman-Sacks endocarditis in patients with SLE was associated with a higher risk for embolic CVD. This suggests that Libman-Sacks endocarditis may be a source of cerebral emboli.
Keywords: CVD; Libman-Sacks endocarditis; MRA; NPSLE; SLE; TEE; TIA; TTE; cerebrovascular disease; magnetic resonance angiography; microembolism; neuropsychiatric systemic lupus erythematosus; stroke; systemic lupus erythematosus; transesophageal echocardiography; transient ischemic attack; transthoracic echocardiography.
Copyright © 2013 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Multimodality imaging, the wave of the future: multidiscipline attack on a complex disease.JACC Cardiovasc Imaging. 2013 Sep;6(9):984-6. doi: 10.1016/j.jcmg.2013.06.004. JACC Cardiovasc Imaging. 2013. PMID: 24029369 No abstract available.
References
-
- The American College of Rheumatology nomenclature and case definitions for neropsychiatric lupus erythematosus. Arthritis Rheum. 1999;42:599–608. - PubMed
-
- Luyendijk J, Steens SC, Ouwendijk WJ, et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum. 2011;63:722–732. - PubMed
-
- Sibbitt WL, Jr, Schmidt PJ, Hart BL, Brooks WM. Fluid Attenuated Inversion Recovery (FLAIR) imaging in neuropsychiatric systemic lupus erythematosus. J Rheumatol. 2003;30:1983–1989. - PubMed
-
- Bernatsky S, Clarke A, Gladman DD, et al. Mortality related to cerebrovascular disease in systemic lupus erythematosus. Lupus. 2006;15:835–839. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

