Stress granules and cell signaling: more than just a passing phase?
- PMID: 24029419
- PMCID: PMC3832949
- DOI: 10.1016/j.tibs.2013.07.004
Stress granules and cell signaling: more than just a passing phase?
Abstract
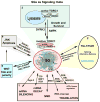
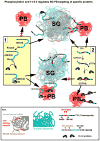
Stress granules (SGs) contain translationally-stalled mRNAs, associated preinitiation factors, and specific RNA-binding proteins. In addition, many signaling proteins are recruited to SGs and/or influence their assembly, which is transient, lasting only until the cells adapt to stress or die. Beyond their role as mRNA triage centers, we posit that SGs constitute RNA-centric signaling hubs analogous to classical multiprotein signaling domains such as transmembrane receptor complexes. As signaling centers, SG formation communicates a 'state of emergency', and their transient existence alters multiple signaling pathways by intercepting and sequestering signaling components. SG assembly and downstream signaling functions may require a cytosolic phase transition facilitated by intrinsically disordered, aggregation-prone protein regions shared by RNA-binding and signaling proteins.
Keywords: cell signaling; intrinsically disordered; protein aggregation; stress granules; translation.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




References
-
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends in biochemical sciences. 2008;33:141–50. - PubMed
-
- Harding H, Novoa Y, Zhang H, Zeng R, Wek M, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108. - PubMed
-
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. - PubMed
-
- Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA- dependent protein kinase. The Journal of biological chemistry. 1998;273:2416–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

