Making a living while starving in the dark: metagenomic insights into the energy dynamics of a carbonate cave
- PMID: 24030597
- PMCID: PMC3906820
- DOI: 10.1038/ismej.2013.159
Making a living while starving in the dark: metagenomic insights into the energy dynamics of a carbonate cave
Abstract
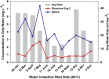
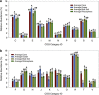
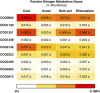
Carbonate caves represent subterranean ecosystems that are largely devoid of phototrophic primary production. In semiarid and arid regions, allochthonous organic carbon inputs entering caves with vadose-zone drip water are minimal, creating highly oligotrophic conditions; however, past research indicates that carbonate speleothem surfaces in these caves support diverse, predominantly heterotrophic prokaryotic communities. The current study applied a metagenomic approach to elucidate the community structure and potential energy dynamics of microbial communities, colonizing speleothem surfaces in Kartchner Caverns, a carbonate cave in semiarid, southeastern Arizona, USA. Manual inspection of a speleothem metagenome revealed a community genetically adapted to low-nutrient conditions with indications that a nitrogen-based primary production strategy is probable, including contributions from both Archaea and Bacteria. Genes for all six known CO2-fixation pathways were detected in the metagenome and RuBisCo genes representative of the Calvin-Benson-Bassham cycle were over-represented in Kartchner speleothem metagenomes relative to bulk soil, rhizosphere soil and deep-ocean communities. Intriguingly, quantitative PCR found Archaea to be significantly more abundant in the cave communities than in soils above the cave. MEtaGenome ANalyzer (MEGAN) analysis of speleothem metagenome sequence reads found Thaumarchaeota to be the third most abundant phylum in the community, and identified taxonomic associations to this phylum for indicator genes representative of multiple CO2-fixation pathways. The results revealed that this oligotrophic subterranean environment supports a unique chemoautotrophic microbial community with potentially novel nutrient cycling strategies. These strategies may provide key insights into other ecosystems dominated by oligotrophy, including aphotic subsurface soils or aquifers and photic systems such as arid deserts.
Figures





References
-
- Banks ED, Taylor NM, Gulley J, Lubbers BR, Giarrizo JG, Bullen HA, et al. Bacterial calcium carbonate precipitation in cave environments: a function of calcium homeostasis. Geomicrobiol J. 2010;27:444–454.
-
- Barton HA, Northup DE. Geomicrobiology in cave environments: past, current and future perspectives. J Cave Karst Stud. 2007;69:163–178.
-
- Barton HA, Taylor MR, Pace NR. Molecular phylogenetic analysis of a bacterial community in an oligotrophic cave environment. Geomicrobiol J. 2004;21:11–20.
-
- Barton HA, Taylor NM, Kreate MP, Springer AC, Oehrle SA, Bertog JL. The impact of host rock geochemistry on bacterial community structure in oligotrophic cave environments. Int J Speleol. 2007;36:93–104.
-
- Bartosch S, Hartwig C, Spieck E, Bock E. Immunological detection of Nitrospira-like bacteria in various soils. Microb Ecol. 2002;43:26–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

