On the way towards greener transition-metal-catalyzed processes as quantified by E factors
- PMID: 24030905
- PMCID: PMC4068147
- DOI: 10.1002/anie.201302020
On the way towards greener transition-metal-catalyzed processes as quantified by E factors
Abstract
Transition-metal-catalyzed carbon-carbon and carbon-heteroatom bond formations are among the most heavily used types of reactions in both academic and industrial settings. As important as these are to the synthetic community, such cross-couplings come with a heavy price to our environment, and sustainability. E Factors are one measure of waste created, and organic solvents, by far, are the main contributors to the high values associated, in particular, with the pharmaceutical and fine-chemical companies which utilize these reactions. An alternative to organic solvents in which cross-couplings are run can be found in the form of micellar catalysis, wherein nanoparticles composed of newly introduced designer surfactants enable the same cross-couplings, albeit in water, with most taking place at room temperature. In the absence of an organic solvent as the reaction medium, organic waste and hence, E Factors, drop dramatically.
Keywords: E Factors; cross-coupling; green chemistry; micelles; water chemistry.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




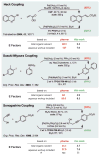



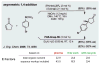
Similar articles
-
Bridging the gap between transition metal- and bio-catalysis via aqueous micellar catalysis.Nat Commun. 2019 May 15;10(1):2169. doi: 10.1038/s41467-019-09751-4. Nat Commun. 2019. PMID: 31092815 Free PMC article.
-
Catalysis in the Service of Green Chemistry: Nobel Prize-Winning Palladium-Catalysed Cross-Couplings, Run in Water at Room Temperature: Heck, Suzuki-Miyaura and Negishi reactions carried out in the absence of organic solvents, enabled by micellar catalysis.Platin Met Rev. 2012 Apr;56(2):62-74. doi: 10.1595/147106712x629761. Platin Met Rev. 2012. PMID: 23555153 Free PMC article.
-
Transition metal-catalyzed efficient and green transformations of P(O)-H compounds to functional organophosphorus compounds.Mini Rev Med Chem. 2013 May 1;13(6):824-35. doi: 10.2174/1389557511313060005. Mini Rev Med Chem. 2013. PMID: 23544461 Review.
-
Water as a solvent: transition metal catalyzed dehydrogenation of alcohols going green.Dalton Trans. 2022 Aug 16;51(32):11987-12020. doi: 10.1039/d2dt01060g. Dalton Trans. 2022. PMID: 35894592 Review.
-
Organocatalytic reactions in water.Chem Commun (Camb). 2009 Nov 28;(44):6687-703. doi: 10.1039/b910861k. Epub 2009 Sep 2. Chem Commun (Camb). 2009. PMID: 19885454 Review.
Cited by
-
Surface-catalyzed hydrolysis by pyrogenic carbonaceous matter and model polymers: An experimental and computational study on functional group and pore characteristics.Appl Catal B. 2022 Dec 15;319:121877. doi: 10.1016/j.apcatb.2022.121877. Epub 2022 Sep 6. Appl Catal B. 2022. PMID: 37846345 Free PMC article.
-
Aerobic oxidation in nanomicelles of aryl alkynes, in water at room temperature.Angew Chem Int Ed Engl. 2014 Mar 24;53(13):3432-5. doi: 10.1002/anie.201310634. Epub 2014 Feb 24. Angew Chem Int Ed Engl. 2014. PMID: 24616243 Free PMC article.
-
Dehalogenation of Functionalized Alkyl Halides in Water at Room Temperature.Green Chem. 2015;2015:307. doi: 10.1039/C4GC01733A. Green Chem. 2015. PMID: 25530711 Free PMC article.
-
Asymmetric gold-catalyzed lactonizations in water at room temperature.Angew Chem Int Ed Engl. 2014 Sep 26;53(40):10658-62. doi: 10.1002/anie.201404729. Epub 2014 Aug 14. Angew Chem Int Ed Engl. 2014. PMID: 25124085 Free PMC article.
-
Fast, greener and scalable direct coupling of organolithium compounds with no additional solvents.Nat Commun. 2016 Jun 2;7:11698. doi: 10.1038/ncomms11698. Nat Commun. 2016. PMID: 27251636 Free PMC article.
References
-
-
For BASF-related, see: Landsiedel R, Saling P. Int J Life Cycle Assess. 2002;7:261.For an alternative discussion, see: Van Aken K, Strekowski L, Patiny L. Beilstein J Org Chem. 2006;2:3.
-
-
- Feuerherd K-H. Macromol Symp. 2003;201:253.
-
- Shonnard DR, Kicherer A, Saling P. Environ Sci Technol. 2003;37:5340. - PubMed
-
- Wall-Markowski CA, Kicherer A, Saling P. Environ Prog. 2004;23:329.
-
- Saling P. Appl Microbiol Biotechnol. 2005;68:1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

