Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock
- PMID: 24031018
- PMCID: PMC3931427
- DOI: 10.1126/science.1240988
Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock
Abstract
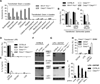
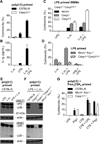
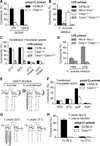
Inflammatory caspases, such as caspase-1 and -11, mediate innate immune detection of pathogens. Caspase-11 induces pyroptosis, a form of programmed cell death, and specifically defends against bacterial pathogens that invade the cytosol. During endotoxemia, however, excessive caspase-11 activation causes shock. We report that contamination of the cytoplasm by lipopolysaccharide (LPS) is the signal that triggers caspase-11 activation in mice. Specifically, caspase-11 responds to penta- and hexa-acylated lipid A, whereas tetra-acylated lipid A is not detected, providing a mechanism of evasion for cytosol-invasive Francisella. Priming the caspase-11 pathway in vivo resulted in extreme sensitivity to subsequent LPS challenge in both wild-type and Tlr4-deficient mice, whereas Casp11-deficient mice were relatively resistant. Together, our data reveal a new pathway for detecting cytoplasmic LPS.
Figures

Comment in
-
Immunology. Sensing endotoxins from within.Science. 2013 Sep 13;341(6151):1184-5. doi: 10.1126/science.1243939. Science. 2013. PMID: 24031006 Free PMC article. No abstract available.
-
When LPS sneaks into the cell.Nat Rev Immunol. 2013 Nov;13(11):772-3. doi: 10.1038/nri3546. Epub 2013 Sep 30. Nat Rev Immunol. 2013. PMID: 24077101 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

