Properties of an amylase from thermophilic Bacillus SP
- PMID: 24031188
- PMCID: PMC3768357
- DOI: 10.1590/S1517-838220080001000023
Properties of an amylase from thermophilic Bacillus SP
Abstract
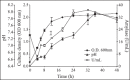
α-Amylase production by thermophilic Bacillus sp strain SMIA-2 cultivated in liquid cultures containing soluble starch as a carbon source and supplemented with 0.05% whey protein and 0.2% peptone reached a maximum activity at 32 h, with levels of 37 U/mL. Studies on the amylase characterization revealed that the optimum temperature of this enzyme was 90°C. The enzyme was stable for 1 h at temperatures ranging from 40-50°C while at 90°C, 66% of its maximum activity was lost. However, in the presence of 5 mM CaCl2, the enzyme was stable at 90°C for 30 min and retained about 58% residual activity after 1 h. The optimum pH of the enzyme was found to be 8.5. After incubation of enzyme for 2 h at pH 9.5 and 11.0 was observed a decrease of about 6.3% and 16.5% of its original activity. At pH 6.0 the enzyme lost about 36% of its original activity. The enzyme was strongly inhibited by Co(2+), Cu(2+) and Ba(2+), but less affected by Mg(2+), Na(+) and K(+). In the presence of 2.0 M NaCl, 63% of amylase activity was retained after 2 h incubation at 45°C. The amylase exhibited more than 70% activity when incubated for 1 h at 50°C with sodium dodecyl sulphate. However, very little residual activity was obtained with sodium hypochlorite and with hydrogen peroxide the enzyme was completely inhibited. The compatibility of Bacillus sp SMIA-2 amylase with certain commercial detergents was shown to be good as the enzyme retained 86%, 85% and 75% of its activity after 20 min incubation at 50°C in the presence of the detergent brands Omo(®), Campeiro(®) and Tide(®), respectively.
Keywords: Bacillus sp; Thermophilic bacterium; α-Amylase.
Figures







References
-
- Aquino A.C.M.M., Jorge J.A., Terenzi H.F., Polizeli M.L.T.M. Studies on a thermostable α-amylase from the thermophilic fungus Scytalidium thermophilum. Appl. Microbiol. Biotechnol. 2003;61:323–328. - PubMed
-
- Banerjee U.C., Sani R.K., Azmi W., Soni R. Thermoestable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem. 1999;35:213–219.
-
- Bernhardsdotter E.C.M.J., Ng J.D., Garriott O.K., Pusey M.L. Enzymic properties of an alkaline chelator-resistant α-amylase from an alkaliphilic Bacillus sp. isolate L1711. Process Biochem. 2005;40:2401–2408.
-
- Bertoldo C., Antranikian G. Starch hydrolyzing enzymes from thermophilic archae and bacteria. Curr. Opin. Chem. Biol. 2002;6:151–160. - PubMed
-
- Bon E.P.S. A tecnologia enzimática no Brasil. ENZITEC. 1995;95:9–14.
LinkOut - more resources
Full Text Sources
Other Literature Sources
