Human T-Lymphotropic Virus (HTLV) Type I in vivo Integration in Oral Keratinocytes
- PMID: 24031637
- PMCID: PMC3768932
- DOI: 10.1590/S1517-83822011000100040
Human T-Lymphotropic Virus (HTLV) Type I in vivo Integration in Oral Keratinocytes
Abstract
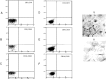
Although the infection of HTLV-1 to cell components of the mouth have been previously reported, there was not until this report, a detailed study to show the characteristics of such infection. From 14 Tropical Spastic Paraparesis/HTLV-1-Associated Myelopathy (HAM/TSP) patients and 11 asymptomatic carrier individuals (AC) coming from HTLV-1 endemic areas of southwest Pacific of Colombia, infected oral mucosa cells were primary cultured during five days. These cell cultures were immunophenotyped by dual color fluorescence cell assortment using different lymphocyte CD markers and also were immunohistochemically processed using a polyclonal anti-keratin antibody. Five days old primary cultures were characterized as oral keratinocytes, whose phenotype was CD3- /CD4-/CD8-/CD19-/CD14-/CD45-/A575-keratin+. From DNA extracted of primary cultures LTR, pol, env and tax HTLV-1 proviral DNA regions were differentially amplified by PCR showing proviral integration. Using poly A+ RNA obtained of these primary cultures, we amplify by RT-PCR cDNA of tax and pol in 57.14% (8/14) HAM/TSP patients and 27.28% (3/11) AC. Tax and pol poly A+ RNA were expressed only in those sIgA positive subjects. Our results showed that proviral integration and viral gene expression in oral keratinocytes are associated with a HTLV-1 specific local mucosal immune response only in those HTLV-1 infected individuals with detectable levels of sIgA in their oral fluids. Altogether the results gave strong evidence that oral mucosa infection would be parte of the systemic spreading of HTLV-1 infection.
Keywords: Human T-Lymphotropic virus type 1; Oral keratinocytes; Oral mucosa; Proviral integration; sIgA.
Figures




References
-
- Achiron, A.; Higuchi, I.; Takenouchi, N.; Matsuok, E.; Hashimoto, K.; Izumo, S.; Shohat, B.; Osame, M. (1997). Detection of HTLV type I provirus by in situ polymerase chain reaction in mouthwash mononuclear cells of HAM/TSP patients and HTLV type I carriers. AIDS Res. Hum. Retrovirus 13,1067-1070. - PubMed
-
- Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254. - PubMed
-
- Carles, G.; Tortevoye, P.; Tuppin, P.; Ureta-Vidal, A.; Peneau, C.; El Guindi, W.; Gessain, A. HTLV1 infection and pregnancy. J Gynecol Obstet Biol Reprod (Paris). 2004;33:14-20. - PubMed
-
- Franchini, G.; Mann, D.L.; Popovic, M.; Zicht, R.R.; Gallo, R.C.; Wong-Staal, F. (1985). HTLV-1 infection of T and B cells of a patient with adult T-cell leukemia-lymphoma (ATLL) and transmission of HTLV-1 from B cells to normal T cells. Leuk. Res 9(11), 1305-1314. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
