Optimization of extracellular thermophilic highly alkaline lipase from thermophilic bacillus sp isolated from hotspring of Arunachal Pradesh, India
- PMID: 24031801
- PMCID: PMC3768969
- DOI: 10.1590/S1517-83822012000100004
Optimization of extracellular thermophilic highly alkaline lipase from thermophilic bacillus sp isolated from hotspring of Arunachal Pradesh, India
Abstract
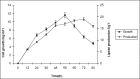
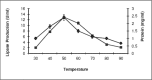
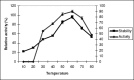
Studies on lipase production were carried out with a bacterial strain (Bacillus sp LBN 2) isolated from soil sample of hotspring of Arunachal Pradesh, India. The cells were cultivated in a mineral medium with maximum production at 1% groundnut oil. The optimum temperature and initial medium pH for lipase production by the organism were 50(0)C and 9.0 respectively. The molecular mass was found to be 33KDa by SDS PAGE. The optimal pH and temperature for activity were 10 and 60(0)C respectively. The enzyme was found to be stable in the pH range of 8-11 with 90% retention of activity at pH 11. The enzyme retained 90% activity at 60(0)C and 70% of activity at 70(0)C for 1h. The lipase was found to be stable in acetone followed by ethanol. The present findings suggested the enzyme to be thermophilic alkaline lipase.
Keywords: Alkaline lipase and Bacillus sp; Thermophilic bacteria; Thermostable.
Figures






References
-
- Abdel-Fattah Y.R. Optimization of thermostable lipase production from a thermophilic Geobacillus sp. using Box-Behnken experimental design. Biotechnol Lett. 2002;24:1217–1222.
-
- Ateslier Z.B.B., Metin K. Production and partial characterization of novel thermostable esterase from a thermophilc Bacillus sp. Enzyme Microb Technol. 2006;38:628–635.
-
- Baral A., Fox P.F. Isolation and characterization of an extracellular lipase from Pseudomonas tolaassi. Fd.. Chem. 1997;58:33–38.
-
- Bayoumi R.A., El-louboudey S.S., Sidkey N.M., Abd-El-Rahman M.A. Production, Purification and Characterization of Thermoalkalophilic Lipase for Application in Bio-detergent Industry. J. Apll. Sci. Res. 2007;3:1752–1765.
LinkOut - more resources
Full Text Sources
