Disease-modifying therapy in multiple sclerosis and chronic inflammatory demyelinating polyradiculoneuropathy: common and divergent current and future strategies
- PMID: 24032475
- PMCID: PMC3927897
- DOI: 10.1111/cei.12195
Disease-modifying therapy in multiple sclerosis and chronic inflammatory demyelinating polyradiculoneuropathy: common and divergent current and future strategies
Abstract
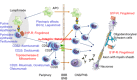
Multiple sclerosis (MS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) represent chronic, autoimmune demyelinating disorders of the central and peripheral nervous system. Although both disorders share some fundamental pathogenic elements, treatments do not provide uniform effects across both disorders. We aim at providing an overview of current and future disease-modifying strategies in these disorders to demonstrate communalities and distinctions. Intravenous immunoglobulins (IVIG) have demonstrated short- and long-term beneficial effects in CIDP but are not effective in MS. Dimethyl fumarate (BG-12), teriflunomide and laquinimod are orally administered immunomodulatory drugs that are already approved or likely to be approved in the near future for the basic therapy of patients with relapsing-remitting MS (RRMS) due to positive results in Phase III clinical trials. However, clinical trials with these drugs in CIDP have not (yet) been initiated. Natalizumab and fingolimod are approved for the treatment of RRMS, and trials to evaluate their safety and efficacy in CIDP are now planned. Alemtuzumab, ocrelizumab and daclizumab respresent monoclonal antibodies in advanced stages of clinical development for their use in RRMS patients. Attempts to study the safety and efficacy of alemtuzumab and B cell-depleting anti-CD20 antibodies, i.e. rituximab, ocrelizumab or ofatumumab, in CIDP patients are currently under way. We provide an overview of the mechanism of action and clinical data available on disease-modifying immunotherapy options for MS and CIDP. Enhanced understanding of the relative effects of therapies in these two disorders may aid rational treatment selection and the development of innovative treatment approaches in the future.
Keywords: CIDP; immunosuppression; immunotherapy; multiple sclerosis.
© 2013 British Society for Immunology.
Figures
References
-
- Koller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343–1356. - PubMed
-
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. - PubMed
-
- Hughes RA, Mehndiratta MM. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2012;(8) CD002062. - PubMed
-
- Mehndiratta MM, Hughes RA. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2012;(9) CD003906. - PubMed
-
- Ciccone A, Beretta S, Brusaferri F, Galea I, Protti A, Spreafico C. Corticosteroids for the long-term treatment in multiple sclerosis. Cochrane Database Syst Rev. 2008;(1) CD006264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical