Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus
- PMID: 24033258
- PMCID: PMC3869896
- DOI: 10.1111/1365-2656.12131
Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus
Abstract
Similar to other infectious diseases, the prevalence of low pathogenic avian influenza viruses (LPAIV) has been seen to exhibit marked seasonal variation. However, mechanisms driving this variation in wild birds have yet to be tested. We investigated the validity of three previously suggested drivers for the seasonal dynamics in LPAIV infections in wild birds: (i) host density, (ii) immunologically naïve young and (iii) increased susceptibility in migrants. To address these questions, we sampled a key LPAIV host species, the mallard Anas platyrhynchos, on a small spatial scale, comprehensively throughout a complete annual cycle, measuring both current and past infection (i.e. viral and seroprevalence, respectively). We demonstrate a minor peak in LPAIV prevalence in summer, a dominant peak in autumn, during which half of the sampled population was infected, and no infections in spring. Seroprevalence of antibodies to a conserved gene segment of avian influenza virus (AIV) peaked in winter and again in spring. The summer peak of LPAIV prevalence coincided with the entrance of unfledged naïve young in the population. Moreover, juveniles were more likely to be infected, shed higher quantities of virus and were less likely to have detectable antibodies to AIV than adult birds. The arrival of migratory birds, as identified by stable hydrogen isotope analysis, appeared to drive the autumn peak in LPAIV infection, with both temporal coincidence and higher infection prevalence in migrants. Remarkably, seroprevalence in migrants was substantially lower than viral prevalence throughout autumn migration, further indicating that each wave of migrants amplified local AIV circulation. Finally, while host abundance increased throughout autumn, it peaked in winter, showing no direct correspondence with either of the LPAIV infection peaks. At an epidemiologically relevant spatial scale, we provide strong evidence for the role of migratory birds as key drivers for seasonal epizootics of LPAIV, regardless of their role as vectors of these viruses. This study exemplifies the importance of understanding host demography and migratory behaviour when examining seasonal drivers of infection in wildlife populations.
Keywords: CT-value; age; infection intensity; infectious disease; nucleoprotein; origin; subtype; viral prevalence.
© 2013 The Authors. Journal of Animal Ecology © 2013 British Ecological Society.
Figures


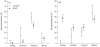

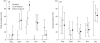
References
-
- Alexander DJ. A review of avian influenza in different bird species. Veterinary Microbiology. 2000;74:3–13. - PubMed
-
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Seasonality and the dynamics of infectious diseases. Ecology Letters. 2006;9:467–484. - PubMed
-
- Altizer S, Bartel R, Han BA. Animal migration and infectious disease risk. Science. 2011;331:296–302. - PubMed
-
- Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, Cannon DL, Khristova ML, Atimnedi P, Paddock CD, Crockett RJK, Flietstra TD, Warfield KL, Unfer R, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Rollin PE, Ksiazek TG, Nichol ST, Towner JS. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. Plos Pathogens. 2012;8:e1002877. - PMC - PubMed
-
- Barlow ND. A spatially aggregated disease host model for bovine Tb in New Zealand possum populations. Journal of Applied Ecology. 1991;28:777–793.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

