Competition between isoprene emission and pigment synthesis during leaf development in aspen
- PMID: 24033429
- PMCID: PMC4411569
- DOI: 10.1111/pce.12190
Competition between isoprene emission and pigment synthesis during leaf development in aspen
Abstract
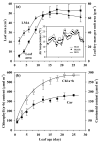
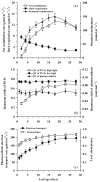
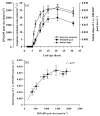
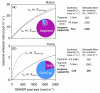
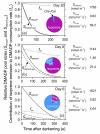
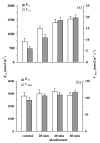
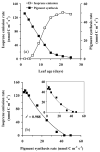
In growing leaves, lack of isoprene synthase (IspS) is considered responsible for delayed isoprene emission, but competition for dimethylallyl diphosphate (DMADP), the substrate for both isoprene synthesis and prenyltransferase reactions in photosynthetic pigment and phytohormone synthesis, can also play a role. We used a kinetic approach based on post-illumination isoprene decay and modelling DMADP consumption to estimate in vivo kinetic characteristics of IspS and prenyltransferase reactions, and to determine the share of DMADP use by different processes through leaf development in Populus tremula. Pigment synthesis rate was also estimated from pigment accumulation data and distribution of DMADP use from isoprene emission changes due to alendronate, a selective inhibitor of prenyltransferases. Development of photosynthetic activity and pigment synthesis occurred with the greatest rate in 1- to 5-day-old leaves when isoprene emission was absent. Isoprene emission commenced on days 5 and 6 and increased simultaneously with slowing down of pigment synthesis. In vivo Michaelis-Menten constant (Km ) values obtained were 265 nmol m(-2) (20 μm) for DMADP-consuming prenyltransferase reactions and 2560 nmol m(-2) (190 μm) for IspS. Thus, despite decelerating pigment synthesis reactions in maturing leaves, isoprene emission in young leaves was limited by both IspS activity and competition for DMADP by prenyltransferase reactions.
Keywords: competition within isoprenoid synthesis pathway; geranyl diphosphate synthase; isoprenoid synthesis; photosynthesis development; prenyltransferase reactions; terpenoid synthesis.
© 2013 John Wiley & Sons Ltd.
Figures

References
-
- Atkinson R, Arey J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmospheric Environment. 2003;37:197–219.
-
- Beisel KG, Schurr U, Matsubara S. Altered turnover of β-carotene and chl a in Arabidopsis leaves treated with lincomycin or norflurazon. Plant and Cell Physiology. 2011;52:1193–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

