Combined γ-tocotrienol and Met inhibitor treatment suppresses mammary cancer cell proliferation, epithelial-to-mesenchymal transition and migration
- PMID: 24033536
- PMCID: PMC6495965
- DOI: 10.1111/cpr.12059
Combined γ-tocotrienol and Met inhibitor treatment suppresses mammary cancer cell proliferation, epithelial-to-mesenchymal transition and migration
Abstract
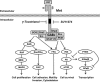
Objectives: Dysregulation of Met signalling is associated with malignant transformation. Combined treatment has been shown to reduce Met activation and mammary tumour cell proliferation. Experiments here, were conducted to determine mechanisms involved in mediating anti-cancer effects of combined γ-tocotrienol and SU11274 (Met inhibitor) treatment in various mammary cancer cell lines.
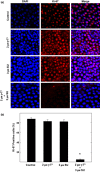
Materials and methods: Treatment effects on mouse (+SA) and human (MCF-7, and MDA-MB-231) mammary cancer cell lines, and normal mouse (CL-S1) and human (MCF10A) mammary epithelial cell lines were compared. Cell proliferation and survival were determined by MTT assay and Ki-67 staining; protein expression was determined by western blot analysis. Immunofluorescence staining was also used to characterize expression and localization of multiple epithelial and mesenchymal markers. Cell migration was determined using a wound-healing assay.
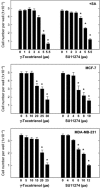
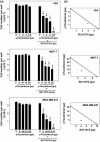
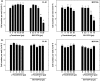
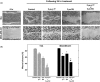
Results: Combined treatment with γ-tocotrienol and SU11274 resulted in synergistic inhibition of +SA, MCF-7, and MDA-MB-231, but not CL-S1 or MCF10A cell growth that was associated with reduction in Akt STAT1/5 and NFκB activation and corresponding blockade in epithelial-to-mesenchymal transition, as indicated by increased expression of E-cadherin, β-catenin, and cytokeratins 8/18 (epithelial markers) and corresponding reduction in vimentin (mesenchymal marker) and reduction in cancer cell motility.
Conclusions: Suggest that combined γ-tocotrienol and Met inhibitor treatment may provide benefit in treatment of breast cancers characterized by aberrant Met activity.
© 2013 John Wiley & Sons Ltd.
Figures


References
-
- Geho DH, Bandle RW, Clair T, Liotta LA (2005) Physiological mechanisms of tumor‐cell invasion and migration. Physiology (Bethesda) 20, 194–200. - PubMed
-
- Porter AC, Vaillancourt RR (1998) Tyrosine kinase receptor‐activated signal transduction pathways which lead to oncogenesis. Oncogene 17, 1343–1352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

