Hyperforin inhibits cell proliferation and differentiation in mouse embryonic stem cells
- PMID: 24033566
- PMCID: PMC6495937
- DOI: 10.1111/cpr.12060
Hyperforin inhibits cell proliferation and differentiation in mouse embryonic stem cells
Abstract
Objectives: Hyperforin, a phloroglucinol derivative of St. John's Wort, has been identified as the major molecule responsible for this plant's products anti-depressant effects. It can be expected that exposure to St. John's Wort during pregnancy occurs with some frequency although embryotoxic or teratogenic effects of St. John's Wort and hyperforin have not yet been experimentally examined in detail. In this study, to determine any embryotoxic effects of hyperforin, we have attempted to determine whether hyperforin affects growth and survival processes of employing mouse embryonic stem (mES) cells (representing embryonic tissue) and fibroblasts (representing adult tissues).
Materials and methods: We used a modified embryonic stem cell test, which has been validated as an in vitro developmental toxicity protocol, mES cells, to assess embryotoxic potential of chemicals under investigation.
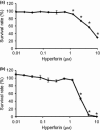
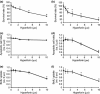
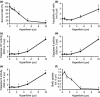
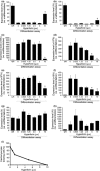
Results: We have identified that high concentrations of hyperforin inhibited mouse ES cell population growth and induced apoptosis in fibroblasts. Under our cell culture conditions, ES cells mainly differentiated into cardiomyocytes, although various other cell types were also produced. In this condition, hyperforin affected ES cell differentiation into cardiomyocytes in a dose-dependent manner. Analysis of tissue-specific marker expression also revealed that hyperforin at high concentrations partially inhibited ES cell differentiation into mesodermal and endodermal lineages.
Conclusions: Hyperforin is currently used in the clinic as a safe and effective antidepressant. Our data indicate that at typical dosages it has only a low risk of embryotoxicity; ingestion of large amounts of hyperforin by pregnant women, however, may pose embryotoxic and teratogenic risks.
© 2013 John Wiley & Sons Ltd.
Figures
Similar articles
-
Understanding drug interactions with St John's wort (Hypericum perforatum L.): impact of hyperforin content.J Pharm Pharmacol. 2019 Jan;71(1):129-138. doi: 10.1111/jphp.12858. Epub 2018 Feb 7. J Pharm Pharmacol. 2019. PMID: 29411879 Review.
-
Effect of eluent pH on the HPLC-UV analysis of hyperforin from St. John's Wort (Hypericum perforatum L.).Phytochem Anal. 2006 Mar-Apr;17(2):71-7. doi: 10.1002/pca.888. Phytochem Anal. 2006. PMID: 16634282
-
Role of hyperforin in the pharmacological activities of St. John's Wort.CNS Drug Rev. 2004 Fall;10(3):203-18. doi: 10.1111/j.1527-3458.2004.tb00022.x. CNS Drug Rev. 2004. PMID: 15492771 Free PMC article. Review.
-
Downregulation of β1 -adrenergic receptors in rat C6 glioblastoma cells by hyperforin and hyperoside from St John's wort.J Pharm Pharmacol. 2013 Jun;65(6):907-15. doi: 10.1111/jphp.12050. Epub 2013 Mar 18. J Pharm Pharmacol. 2013. PMID: 23647684
-
Hyperforin, a bio-active compound of St. John's Wort, is a new inhibitor of angiogenesis targeting several key steps of the process.Int J Cancer. 2005 Dec 10;117(5):775-80. doi: 10.1002/ijc.21246. Int J Cancer. 2005. PMID: 15981212
Cited by
-
Medicinal Plants for the Treatment of Mental Diseases in Pregnancy: An In Vitro Safety Assessment.Planta Med. 2022 Oct;88(12):1036-1046. doi: 10.1055/a-1628-8132. Epub 2021 Oct 8. Planta Med. 2022. PMID: 34624906 Free PMC article.
-
Advanced in Vitro Safety Assessment of Herbal Medicines for the Treatment of Non-Psychotic Mental Disorders in Pregnancy.Front Pharmacol. 2022 Jun 23;13:882997. doi: 10.3389/fphar.2022.882997. eCollection 2022. Front Pharmacol. 2022. PMID: 35814220 Free PMC article.
-
St. John's Wort usage in treating of perinatal depression.Front Behav Neurosci. 2023 Jan 5;16:1066459. doi: 10.3389/fnbeh.2022.1066459. eCollection 2022. Front Behav Neurosci. 2023. PMID: 36688122 Free PMC article. No abstract available.
References
-
- Calixto JB, Campos MM, Otuki MF, Santos AR (2004) Anti‐inflammatory compounds of plant origin. Part II. modulation of pro‐inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 70, 93–103. - PubMed
-
- Calixto JB, Otuki MF, Santos AR (2003) Anti‐inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF‐kappaB). Planta Med. 69, 973–983. - PubMed
-
- Bailly C (2009) Ready for a comeback of natural products in oncology. Biochem. Pharmacol. 77, 1447–1457. - PubMed
-
- Medina MA, Martinez‐Poveda B, Amores‐Sanchez MI, Quesada AR (2006) Hyperforin: more than an antidepressant bioactive compound? Life Sci. 79, 105–111. - PubMed
-
- Quiney C, Billard C, Salanoubat C, Fourneron JD, Kolb JP (2006) Hyperforin, a new lead compound against the progression of cancer and leukemia? Leukemia 20, 1519–1525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

