Glycolytic fast-twitch muscle fiber restoration counters adverse age-related changes in body composition and metabolism
- PMID: 24033924
- PMCID: PMC3947044
- DOI: 10.1111/acel.12153
Glycolytic fast-twitch muscle fiber restoration counters adverse age-related changes in body composition and metabolism
Abstract
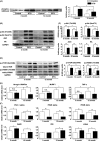
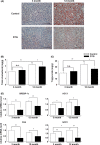
Aging is associated with the development of insulin resistance, increased adiposity, and accumulation of ectopic lipid deposits in tissues and organs. Starting in mid-life there is a progressive decline in lean muscle mass associated with the preferential loss of glycolytic, fast-twitch myofibers. However, it is not known to what extent muscle loss and metabolic dysfunction are causally related or whether they are independent epiphenomena of the aging process. Here, we utilized a skeletal-muscle-specific, conditional transgenic mouse expressing a constitutively active form of Akt1 to examine the consequences of glycolytic, fast-twitch muscle growth in young vs. middle-aged animals fed standard low-fat chow diets. Activation of the Akt1 transgene led to selective skeletal muscle hypertrophy, reversing the loss of lean muscle mass observed upon aging. The Akt1-mediated increase in muscle mass led to reductions in fat mass and hepatic steatosis in older animals, and corrected age-associated impairments in glucose metabolism. These results indicate that the loss of lean muscle mass is a significant contributor to the development of age-related metabolic dysfunction and that interventions that preserve or restore fast/glycolytic muscle may delay the onset of metabolic disease.
Keywords: adipose tissue; diabetes; exercise; mTOR; sarcopenia; type IIb muscle.
© 2013 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures




References
-
- Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. - PubMed
-
- Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A. Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J. Biol. Chem. 2008;283:4228–4240. - PubMed
-
- Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl. Acad. Sci. U S A. 2008;105:19926–19931. - PMC - PubMed
-
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. - PubMed
-
- Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994;330:1769–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

