Molecular structure of β-amyloid fibrils in Alzheimer's disease brain tissue
- PMID: 24034249
- PMCID: PMC3814033
- DOI: 10.1016/j.cell.2013.08.035
Molecular structure of β-amyloid fibrils in Alzheimer's disease brain tissue
Abstract
In vitro, β-amyloid (Aβ) peptides form polymorphic fibrils, with molecular structures that depend on growth conditions, plus various oligomeric and protofibrillar aggregates. Here, we investigate structures of human brain-derived Aβ fibrils, using seeded fibril growth from brain extract and data from solid-state nuclear magnetic resonance and electron microscopy. Experiments on tissue from two Alzheimer's disease (AD) patients with distinct clinical histories showed a single predominant 40 residue Aβ (Aβ40) fibril structure in each patient; however, the structures were different from one another. A molecular structural model developed for Aβ40 fibrils from one patient reveals features that distinguish in-vivo- from in-vitro-produced fibrils. The data suggest that fibrils in the brain may spread from a single nucleation site, that structural variations may correlate with variations in AD, and that structure-specific amyloid imaging agents may be an important future goal.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
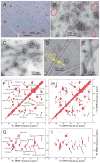
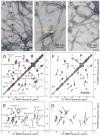
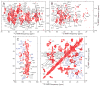
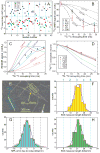

Comment in
-
A template for new drugs against Alzheimer's disease.Cell. 2013 Sep 12;154(6):1182-4. doi: 10.1016/j.cell.2013.08.049. Cell. 2013. PMID: 24034239
-
Amyloid structures from Alzheimer's disease patients.Structure. 2013 Oct 8;21(10):1722-3. doi: 10.1016/j.str.2013.09.010. Structure. 2013. PMID: 24119671 Free PMC article.
References
-
- Bertini I, Gonnelli L, Luchinat C, Mao JF, Nesi A. A new structural model of Aβ40 fibrils. J Am Chem Soc. 2011;133:16013–16022. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

