A Rab-centric perspective of bacterial pathogen-occupied vacuoles
- PMID: 24034612
- PMCID: PMC3839303
- DOI: 10.1016/j.chom.2013.08.010
A Rab-centric perspective of bacterial pathogen-occupied vacuoles
Abstract
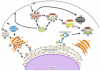
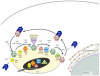
The ability to create and maintain a specialized organelle that supports bacterial replication is an important virulence property for many intracellular pathogens. Living in a membrane-bound vacuole presents inherent challenges, including the need to remodel a plasma membrane-derived organelle into a novel structure that will expand and provide essential nutrients to support replication, while also having the vacuole avoid membrane transport pathways that target bacteria for destruction in lysosomes. It is clear that pathogenic bacteria use different strategies to accomplish these tasks. The dynamics by which host Rab GTPases associate with pathogen-occupied vacuoles provide insight into the mechanisms used by different bacteria to manipulate host membrane transport. In this review we highlight some of the strategies bacteria use to maintain a pathogen-occupied vacuole by focusing on the Rab proteins involved in biogenesis and maintenance of these novel organelles.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol. 2012;14:11–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

