Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease
- PMID: 24034790
- PMCID: PMC3866211
- DOI: 10.1111/pedi.12066
Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease
Abstract
Objective: The Environmental Determinants of Diabetes in the Young (TEDDY) study is designed to identify environmental exposures triggering islet autoimmunity and type 1 diabetes (T1D) in genetically high-risk children. We describe the first 100 participants diagnosed with T1D, hypothesizing that (i) they are diagnosed at an early stage of disease, (ii) a high proportion are diagnosed by an oral glucose tolerance test (OGTT), and (iii) risk for early T1D is related to country, population, human leukocyte antigen (HLA)-genotypes and immunological markers.
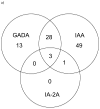
Methods: Autoantibodies to glutamic acid decarboxylase (GADA), insulinoma-associated protein 2 (IA-2) and insulin (IAA) were analyzed from 3 months of age in children with genetic risk. Symptoms and laboratory values at diagnosis were obtained and reviewed for ADA criteria.
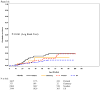
Results: The first 100 children to develop T1D, 33 first-degree relatives (FDRs), with a median age 2.3 yr (0.69-6.27), were diagnosed between September 2005 and November 2011. Although young, 36% had no symptoms and ketoacidosis was rare (8%). An OGTT diagnosed 9/30 (30%) children above 3 yr of age but only 4/70 (5.7%) below the age of 3 yr. FDRs had higher cumulative incidence than children from the general population (p < 0.0001). Appearance of all three autoantibodies at seroconversion was associated with the most rapid development of T1D (HR = 4.52, p = 0.014), followed by the combination of GADA and IAA (HR = 2.82, p < 0.0001).
Conclusions: Close follow-up of children with genetic risk enables early detection of T1D. Risk factors for rapid development of diabetes in this young population were FDR status and initial positivity for GADA, IA-2, and IAA or a combination of GADA and IAA.
Keywords: TEDDY study; autoantibodies; diagnosis; follow-up studies; type 1 diabetes.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures




References
-
- IDF. Diabetes Atlas. 2006:2.
-
- Ludvigsson J. Immune intervention at diagnosis--should we treat children to preserve beta-cell function? Pediatr Diabetes. 2007;8 (Suppl 6):34–9. - PubMed
-
- Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes. 2008;9:197–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK63865/DK/NIDDK NIH HHS/United States
- UC4 DK063863/DK/NIDDK NIH HHS/United States
- U01 DK063836/DK/NIDDK NIH HHS/United States
- DK63790/DK/NIDDK NIH HHS/United States
- UC4 DK063829/DK/NIDDK NIH HHS/United States
- HHSN267200700014C/DK/NIDDK NIH HHS/United States
- DK63863/DK/NIDDK NIH HHS/United States
- DK63861/DK/NIDDK NIH HHS/United States
- UC4 DK063865/DK/NIDDK NIH HHS/United States
- DK63836/DK/NIDDK NIH HHS/United States
- DK63829/DK/NIDDK NIH HHS/United States
- U01 DK063829/DK/NIDDK NIH HHS/United States
- UC4 DK095300/DK/NIDDK NIH HHS/United States
- UC4 DK063821/DK/NIDDK NIH HHS/United States
- UC4DK095300/DK/NIDDK NIH HHS/United States
- UC4 DK063836/DK/NIDDK NIH HHS/United States
- DK63821/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

