Cytoplasmic dynein heavy chain: the servant of many masters
- PMID: 24035135
- PMCID: PMC3824068
- DOI: 10.1016/j.tins.2013.08.001
Cytoplasmic dynein heavy chain: the servant of many masters
Abstract
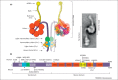
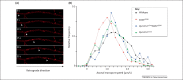
Cytoplasmic dynein is the main retrograde motor in all eukaryotic cells. This complex comprises different subunits assembled on a cytoplasmic dynein heavy chain 1 (DYNC1H1) dimer. Cytoplasmic dynein is particularly important for neurons because it carries essential signals and organelles from distal sites to the cell body. In the past decade, several mouse models have helped to dissect the numerous functions of DYNC1H1. Additionally, several DYNC1H1 mutations have recently been found in human patients that give rise to a broad spectrum of developmental and midlife-onset disorders. Here, we discuss the effects of mutations of mouse and human DYNC1H1 and how these studies are giving us new insight into the many critical roles DYNC1H1 plays in the nervous system.
Keywords: Cramping 1; Legs at odd angles; Sprawling; amyotrophic lateral sclerosis; axonal transport; motor neurons; neurodegeneration.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
-
- Vallee R.B. Multiple modes of cytoplasmic dynein regulation. Nat. Cell Biol. 2012;14:224–230. - PubMed
-
- Kon T. The 2.8 angstrom crystal structure of the dynein motor domain. Nature. 2012;484:345–351. - PubMed
-
- Carter A.P. Crystal clear insights into how the dynein motor moves. J. Cell Sci. 2013;126:705–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

