Modulation of curli assembly and pellicle biofilm formation by chemical and protein chaperones
- PMID: 24035282
- PMCID: PMC4243843
- DOI: 10.1016/j.chembiol.2013.07.017
Modulation of curli assembly and pellicle biofilm formation by chemical and protein chaperones
Abstract
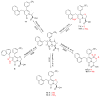
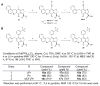
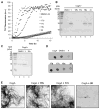
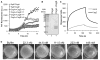
Enteric bacteria assemble functional amyloid fibers, curli, on their surfaces that share structural and biochemical properties with disease-associated amyloids. Here, we test rationally designed 2-pyridone compounds for their ability to alter amyloid formation of the major curli subunit CsgA. We identified several compounds that discourage CsgA amyloid formation and several compounds that accelerate CsgA amyloid formation. The ability of inhibitor compounds to stop growing CsgA fibers was compared to the same property of the CsgA chaperone, CsgE. CsgE blocked CsgA amyloid assembly and arrested polymerization when added to actively polymerizing fibers. Additionally, CsgE and the 2-pyridone inhibitors prevented biofilm formation by Escherichia coli at the air-liquid interface of a static culture. We demonstrate that curli amyloid assembly and curli-dependent biofilm formation can be modulated not only by protein chaperones, but also by "chemical chaperones."
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


References
-
- Ban T, Hamada D, Hasegawa K, Naiki H, Goto Y. Direct observation of amyloid fibril growth monitored by thioflavin T fluorescence. J Biol Chem. 2003;278:16462–16465. - PubMed
-
- Berg V, Sellstedt M, Hedenström M, Pinkner JS, Hultgren SJ, Almqvist F. Design, synthesis and evaluation of peptidomimetics based on substituted bicyclic 2-pyridones-targeting virulence of uropathogenic E. coli. Bioorg Med Chem. 2006;14:7563–7581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

