West Nile viral infection of equids
- PMID: 24035480
- PMCID: PMC4581842
- DOI: 10.1016/j.vetmic.2013.08.013
West Nile viral infection of equids
Abstract
West Nile virus (WNV) is a flavivirus transmitted between certain species of birds and mosquito vectors. Tangential infections of equids and subsequent equine epizootics have occurred historically. Although the attack rate has been estimated to be below 10%, mortality rates can approach 50% in horses that present clinical disease. Symptoms are most commonly presenting in the form of encephalitis with ataxia as well as limb weakness, recumbency and muscle fasciculation. The most effective strategy for prevention of equine disease is proper vaccination with one of the numerous commercially available vaccines available in North America or the European Union. Recently, WNV has been increasingly associated with equine epizootics resulting from novel non-lineage-1a viruses in expanding geographic areas. However, specific experimental data on the virulence of these novel virus strains is lacking and questions remain as to the etiology of the expanded epizootics: whether it be a function of inherent virulence or ecological and/or climactic factors that could precipitate the altered epidemiological patterns observed.
Keywords: Equids; Flavivirus; Horses; West Nile virus.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

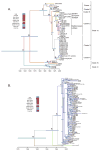
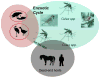



References
-
- APHIS. Annual trends in US distribution of equine West Nile virus cases. APHIS; 2012. West Nile virus distribution maps, 1999–2012. http://www.aphis.usda.gov/vs/nahss/equine/wnv/wnv_distribution_maps.htm.
-
- Bagnarelli P, Marinelli K, Trotta D, Monachetti A, Tavio M, Del Gobbo R, Capobianchi M, Menzo S, Nicoletti L, Magurano F, Varaldo P. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Euro Surveill. 2011;16 pii 20002. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

