Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies
- PMID: 24035558
- PMCID: PMC5015432
- DOI: 10.1016/S1473-3099(13)70252-4
Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies
Abstract
Background: Artemisinin resistance in Plasmodium falciparum lengthens parasite clearance half-life during artemisinin monotherapy or artemisinin-based combination therapy. Absence of in-vitro and ex-vivo correlates of artemisinin resistance hinders study of this phenotype. We aimed to assess whether an in-vitro ring-stage survival assay (RSA) can identify culture-adapted P falciparum isolates from patients with slow-clearing or fast-clearing infections, to investigate the stage-dependent susceptibility of parasites to dihydroartemisinin in the in-vitro RSA, and to assess whether an ex-vivo RSA can identify artemisinin-resistant P falciparum infections.
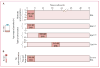
Methods: We culture-adapted parasites from patients with long and short parasite clearance half-lives from a study done in Pursat, Cambodia, in 2010 (registered with ClinicalTrials.gov, number NCT00341003) and used novel in-vitro survival assays to explore the stage-dependent susceptibility of slow-clearing and fast-clearing parasites to dihydroartemisinin. In 2012, we implemented the RSA in prospective parasite clearance studies in Pursat, Preah Vihear, and Ratanakiri, Cambodia (NCT01736319), to measure the ex-vivo responses of parasites from patients with malaria. Continuous variables were compared with the Mann-Whitney U test. Correlations were analysed with the Spearman correlation test.
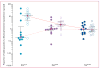
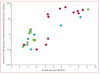
Findings: In-vitro survival rates of culture-adapted parasites from 13 slow-clearing and 13 fast-clearing infections differed significantly when assays were done on 0-3 h ring-stage parasites (10·88% vs 0·23%; p=0·007). Ex-vivo survival rates significantly correlated with in-vivo parasite clearance half-lives (n=30, r=0·74, 95% CI 0·50-0·87; p<0·0001).
Interpretation: The in-vitro RSA of 0-3 h ring-stage parasites provides a platform for the molecular characterisation of artemisinin resistance. The ex-vivo RSA can be easily implemented where surveillance for artemisinin resistance is needed.
Funding: Institut Pasteur du Cambodge and the Intramural Research Program, NIAID, NIH.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare that we have no conflicts of interest.
Figures
Comment in
-
Tracking artemisinin resistance in Plasmodium falciparum.Lancet Infect Dis. 2013 Dec;13(12):999-1000. doi: 10.1016/S1473-3099(13)70260-3. Epub 2013 Sep 11. Lancet Infect Dis. 2013. PMID: 24035557 No abstract available.
-
Artemisinin resistance in Plasmodium falciparum.Lancet Infect Dis. 2014 Jun;14(6):449-50. doi: 10.1016/S1473-3099(14)70777-7. Lancet Infect Dis. 2014. PMID: 24849722 Free PMC article. No abstract available.
-
Artemisinin resistance in Plasmodium falciparum.Lancet Infect Dis. 2014 Jun;14(6):450-1. doi: 10.1016/S1473-3099(14)70706-6. Lancet Infect Dis. 2014. PMID: 24849723 No abstract available.
References
-
- WHO. Antimalarial drug combination therapy. Geneva: World Health Organization; 2001.
-
- WHO. World malaria report 2012. Geneva: World Health Organization; 2012.
-
- Wongsrichanalai C, Pickard AL, Wernsdorfer WH, et al. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–18. - PubMed
-
- Noedl H, Se Y, Schaecher K, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

