Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model
- PMID: 24035612
- PMCID: PMC3814653
- DOI: 10.1016/j.ajpath.2013.07.022
Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model
Abstract
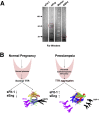
Preeclampsia is a major pregnancy complication with potential short- and long-term consequences for both mother and fetus. Understanding its pathogenesis and causative biomarkers is likely to yield insights for prediction and treatment. Herein, we provide evidence that transthyretin, a transporter of thyroxine and retinol, is aggregated in preeclampsia and is present at reduced levels in sera of preeclamptic women, as detected by proteomic screen. We demonstrate that transthyretin aggregates form deposits in preeclampsia placental tissue and cause apoptosis. By using in vitro approaches and a humanized mouse model, we provide evidence for a causal link between dysregulated transthyretin and preeclampsia. Native transthyretin inhibits all preeclampsia-like features in the humanized mouse model, including new-onset proteinuria, increased blood pressure, glomerular endotheliosis, and production of anti-angiogenic factors. Our findings suggest that a focus on transthyretin structure and function is a novel strategy to understand and combat preeclampsia.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia.Sci Rep. 2017 Jul 27;7(1):6694. doi: 10.1038/s41598-017-07017-x. Sci Rep. 2017. PMID: 28751735 Free PMC article.
-
Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia.Reprod Sci. 2014 Jan;21(1):138-45. doi: 10.1177/1933719113492207. Epub 2013 Jun 7. Reprod Sci. 2014. PMID: 23749761
-
Nicotine increases hepatocyte transthyretin turnover: A possible mechanism for the protective effect of smoking on preeclampsia?Mol Cell Endocrinol. 2025 Feb 1;597:112446. doi: 10.1016/j.mce.2024.112446. Epub 2024 Dec 24. Mol Cell Endocrinol. 2025. PMID: 39725350
-
Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia.Pediatr Res. 2005 May;57(5 Pt 2):1R-7R. doi: 10.1203/01.PDR.0000159567.85157.B7. Epub 2005 Apr 6. Pediatr Res. 2005. PMID: 15817508 Review.
-
Growth factors in preeclampsia: a vascular disease model. A failed vasodilation and angiogenic challenge from pregnancy onwards?Cytokine Growth Factor Rev. 2013 Oct;24(5):411-25. doi: 10.1016/j.cytogfr.2013.05.008. Epub 2013 Jun 22. Cytokine Growth Factor Rev. 2013. PMID: 23800655 Review.
Cited by
-
Thyroid Hormone Distributor Proteins During Development in Vertebrates.Front Endocrinol (Lausanne). 2019 Aug 8;10:506. doi: 10.3389/fendo.2019.00506. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31440205 Free PMC article. Review.
-
Novel blood test for early biomarkers of preeclampsia and Alzheimer's disease.Sci Rep. 2021 Aug 5;11(1):15934. doi: 10.1038/s41598-021-95611-5. Sci Rep. 2021. PMID: 34354200 Free PMC article.
-
Transthyretin increases migration and invasion of rat placental trophoblast cells.FEBS Open Bio. 2020 Aug;10(8):1568-1576. doi: 10.1002/2211-5463.12911. Epub 2020 Jul 1. FEBS Open Bio. 2020. PMID: 32533762 Free PMC article.
-
The Physiological Roles of Amyloid-β Peptide Hint at New Ways to Treat Alzheimer's Disease.Front Aging Neurosci. 2018 Apr 25;10:118. doi: 10.3389/fnagi.2018.00118. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29922148 Free PMC article. Review.
-
Quantitative proteomics-based analyses performed on pre-eclampsia samples in the 2004-2020 period: a systematic review.Clin Proteomics. 2021 Jan 26;18(1):6. doi: 10.1186/s12014-021-09313-1. Clin Proteomics. 2021. PMID: 33499801 Free PMC article. Review.
References
-
- Roberts J.M., Hubel C.A. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354:788–789. - PubMed
-
- Redman C.W., Sargent I.L. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. - PubMed
-
- Dekker G., Sibai B. Primary, secondary and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209–215. - PubMed
-
- Steegers E.A., Dadelszen P., Duvekot J.J., Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. - PubMed
-
- Parikh S.M., Karumanchi S.A. Putting pressure on pre-eclampsia. Nat Med. 2008;14:810–812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

