Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: a critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices
- PMID: 24035904
- PMCID: PMC4380082
- DOI: 10.1093/cercor/bht245
Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: a critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices
Abstract
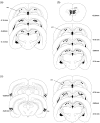
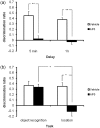
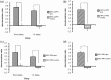
Object-in-place associative recognition memory depends on an interaction between the hippocampus (HPC), perirhinal (PRH), and medial prefrontal (mPFC) cortices, yet the contribution of glutamate receptor neurotransmission to these interactions is unknown. NMDA receptors (NMDAR) in the HPC were critical for encoding of object-in-place memory but not for single-item object recognition. Next, a disconnection procedure was used to examine the importance of "concurrent" glutamate neurotransmission in the HPC-mPFC and HPC-PRH. Contralateral unilateral infusions of NBQX (AMPAR antagonist), into the HPC-mPFC, or HPC-PRH, either before acquisition or test, impaired object-in-place performance. Thus, both circuits are necessary for encoding and retrieval. Crossed unilateral AP5 (NMDAR antagonist) infusions into the HPC-mPFC or HPC-PRH impaired encoding, but not retrieval. Specifically crossed HPC-mPFC infusions impaired both short-term (5 min) and longer term (1 h) memory while HPC-PRH infusions impaired longer term memory only. This delay-dependent effect of AP5 in the HPC-PRH on object-in-place memory, accords with its effects in the PRH, on single item object recognition memory, thereby suggesting that a single PRH synaptic plasticity mechanism underpins different recognition memory processes. Further, blocking excitatory neurotransmission in any pair of structures within the networks impaired "both" encoding and retrieval, thus object-in-place memory clearly requires network interdependency across multiple structures.
Keywords: brain circuits; encoding; glutamate receptors; plasticity; retrieval.
© The Author 2013. Published by Oxford University Press.
Figures

References
-
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cereb Cortex. 2006;16:1759–1765. - PubMed
-
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

