VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy
- PMID: 24036251
- PMCID: PMC3814520
- DOI: 10.1016/j.ajpath.2013.07.031
VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy
Abstract
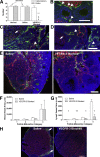
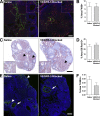
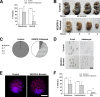
Lymphatic vessels surround follicles within the ovary, but their roles in folliculogenesis and pregnancy, as well as the necessity of lymphangiogenesis in follicle maturation and health, are undefined. We used systemic delivery of mF4-31C1, a specific antagonist vascular endothelial growth factor receptor 3 (VEGFR-3) antibody to block lymphangiogenesis in mice. VEGFR-3 neutralization for 2 weeks before mating blocked ovarian lymphangiogenesis at all stages of follicle maturation, most notably around corpora lutea, without significantly affecting follicular blood angiogenesis. The numbers of oocytes ovulated, fertilized, and implanted in the uterus were normal in these mice; however, pregnancies were unsuccessful because of retarded fetal growth and miscarriage. Fewer patent secondary follicles were isolated from treated ovaries, and isolated blastocysts exhibited reduced cell densities. Embryos from VEGFR-3-neutralized dams developed normally when transferred to untreated surrogates. Conversely, normal embryos transferred into mF4-31C1-treated dams led to the same fetal deficiencies observed with in situ gestation. Although no significant changes were measured in uterine blood or lymphatic vascular densities, VEGFR-3 neutralization reduced serum and ovarian estradiol concentrations during gestation. VEGFR-3-mediated lymphangiogenesis thus appears to modulate the folliculogenic microenvironment and may be necessary for maintenance of hormone levels during pregnancy; both of these are novel roles for the lymphatic vasculature.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
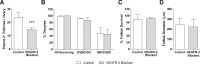
References
-
- Brown H.M., Dunning K.R., Robker R.L., Pritchard M., Russell D.L. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 2006;300:699–709. - PubMed
-
- Brown H.M., Robker R.L., Russell D.L. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology. 2010;151:5446–5455. - PubMed
-
- Gaytán F., Tarradas E., Bellido C., Morales C., Sánchez-Criado J.E. Prostaglandin E(1) inhibits abnormal follicle rupture and restores ovulation in indomethacin-treated rats. Biol Reprod. 2002;67:1140–1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

