Genome signature-based dissection of human gut metagenomes to extract subliminal viral sequences
- PMID: 24036533
- PMCID: PMC3778543
- DOI: 10.1038/ncomms3420
Genome signature-based dissection of human gut metagenomes to extract subliminal viral sequences
Abstract
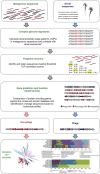
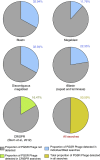
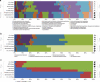
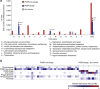
Bacterial viruses (bacteriophages) have a key role in shaping the development and functional outputs of host microbiomes. Although metagenomic approaches have greatly expanded our understanding of the prokaryotic virosphere, additional tools are required for the phage-oriented dissection of metagenomic data sets, and host-range affiliation of recovered sequences. Here we demonstrate the application of a genome signature-based approach to interrogate conventional whole-community metagenomes and access subliminal, phylogenetically targeted, phage sequences present within. We describe a portion of the biological dark matter extant in the human gut virome, and bring to light a population of potentially gut-specific Bacteroidales-like phage, poorly represented in existing virus like particle-derived viral metagenomes. These predominantly temperate phage were shown to encode functions of direct relevance to human health in the form of antibiotic resistance genes, and provided evidence for the existence of putative 'viral-enterotypes' among this fraction of the human gut virome.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

