Endogenous factor VIII synthesis from the intron 22-inverted F8 locus may modulate the immunogenicity of replacement therapy for hemophilia A
- PMID: 24037092
- PMCID: PMC4123441
- DOI: 10.1038/nm.3270
Endogenous factor VIII synthesis from the intron 22-inverted F8 locus may modulate the immunogenicity of replacement therapy for hemophilia A
Abstract
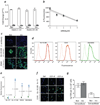
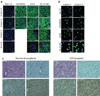
Neutralizing antibodies (inhibitors) to replacement factor VIII (FVIII, either plasma derived or recombinant) impair the effective management of hemophilia A. Individuals with hemophilia A due to major deletions of the FVIII gene (F8) lack antigenically cross-reactive material in their plasma ("CRM-negative"), and the prevalence of inhibitors in these individuals may be as high as 90%. Conversely, individuals with hemophilia A caused by F8 missense mutations are CRM-positive, and their overall prevalence of inhibitors is <10% (ref. 2). Individuals with the F8 intron 22 inversion (found in ∼50% of individuals with severe hemophilia A) have been grouped with the former on the basis of their genetic defect and CRM-negative status. However, only ∼20% of these individuals develop inhibitors. Here we demonstrate that the levels of F8 mRNA and intracellular FVIII protein in B lymphoblastoid cells and liver biopsies from individuals with the intron 22 inversion are comparable to those in healthy controls. These results support the hypothesis that most individuals with the intron 22 inversion are tolerized to FVIII and thus do not develop inhibitors. Furthermore, we developed a new pharmacogenetic algorithm that permits the stratification of inhibitor risk for individuals and subpopulations by predicting the immunogenicity of replacement FVIII using, as input, the number of putative T cell epitopes in the infused protein and the competence of major histocompatibility complex class II molecules to present such epitopes. This algorithm showed statistically significant accuracy in predicting the presence of inhibitors in 25 unrelated individuals with the intron 22 inversion.
Figures


References
-
- Lillicrap D. Improvements in factor concentrates. Curr. Opin. Hematol. 2010;17:393–397. - PubMed
-
- Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9:418–435. - PubMed
-
- Gouw SC, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. 2012;119:2922–2934. - PubMed
-
- Jenkins PV, et al. Analysis of intron 22 inversions of the factor VIII gene in severe hemophilia A: implications for genetic counseling. Blood. 1994;84:2197–2201. - PubMed
-
- Egler C, et al. Kinetic parameters of monoclonal antibodies ESH2, ESH4, ESH5, and ESH8 on coagulation factor VIII and their influence on factor VIII activity. J. Mol. Recognit. 2009;22:301–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

