Comparison of 4D-microSPECT and microCT for murine cardiac function
- PMID: 24037175
- PMCID: PMC4061569
- DOI: 10.1007/s11307-013-0686-z
Comparison of 4D-microSPECT and microCT for murine cardiac function
Abstract
Purpose: The objective of this study was to compare a new generation of four-dimensional micro-single photon emission computed tomography (microSPECT) with microCT for the quantitative in vivo assessment of murine cardiac function.
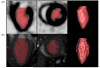
Procedures: Four-dimensional isotropic cardiac images were acquired from anesthetized normal C57BL/6 mice with either microSPECT (n = 6) or microCT (n = 6). One additional mouse with myocardial infarction (MI) was scanned with both modalities. Prior to imaging, mice were injected with either technetium tetrofosmin for microSPECT or a liposomal blood pool contrast agent for microCT. Segmentation of the left ventricle (LV) was performed using Vitrea (Vital Images) software, to derive global and regional function.
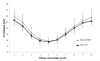
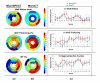
Results: Measures of global LV function between microSPECT and microCT groups were comparable (e.g., ejection fraction = 71 ± 6 % microSPECT and 68 ± 4 % microCT). Regional functional indices (wall motion, wall thickening, regional ejection fraction) were also similar for the two modalities. In the mouse with MI, microSPECT identified a large perfusion defect that was not evident with microCT.
Conclusions: Despite lower spatial resolution, microSPECT was comparable to microCT in the quantitative evaluation of cardiac function. MicroSPECT offers an advantage over microCT in the ability to evaluate simultaneously myocardial radiotracer distribution and function, simultaneously. MicroSPECT should be considered as an alternative to microCT and magnetic resonance for preclinical cardiac imaging in the mouse.
Figures




References
-
- Breckenridge R. Heart failure and mouse models. Dis Models Mech. 2010;3(3-4):138–143. - PubMed
-
- Constantinides C. Study of the Murine Cardiac Mechanical Function Using Magnetic Resonance Imaging: The Current Status, Challenges, and Future Perspectives. In: Andrade A, editor. Practical Applications in Biomedical Engineering. 2013. ISBN: 978-953-51-0924-2, InTech.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

