Localisation of AMPK γ subunits in cardiac and skeletal muscles
- PMID: 24037260
- PMCID: PMC3853370
- DOI: 10.1007/s10974-013-9359-4
Localisation of AMPK γ subunits in cardiac and skeletal muscles
Abstract
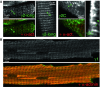
The trimeric protein AMP-activated protein kinase (AMPK) is an important sensor of energetic status and cellular stress, and mutations in genes encoding two of the regulatory γ subunits cause inherited disorders of either cardiac or skeletal muscle. AMPKγ2 mutations cause hypertrophic cardiomyopathy with glycogen deposition and conduction abnormalities; mutations in AMPKγ3 result in increased skeletal muscle glycogen. In order to gain further insight into the roles of the different γ subunits in muscle and into possible disease mechanisms, we localised the γ2 and γ3 subunits, along with the more abundant γ1 subunit, by immunofluorescence in cardiomyocytes and skeletal muscle fibres. The predominant cardiac γ2 variant, γ2-3B, gave a striated pattern in cardiomyocytes, aligning with the Z-disk but with punctate staining similar to T-tubule (L-type Ca(2+) channel) and sarcoplasmic reticulum (SERCA2) markers. In skeletal muscle fibres AMPKγ3 localises to the I band, presenting a uniform staining that flanks the Z-disk, also coinciding with the position of Ca(2+) influx in these muscles. The localisation of γ2-3B- and γ3-containing AMPK suggests that these trimers may have similar functions in the different muscles. AMPK containing γ2-3B was detected in oxidative skeletal muscles which had low expression of γ3, confirming that these two regulatory subunits may be co-ordinately regulated in response to metabolic requirements. Compartmentalisation of AMPK complexes is most likely dependent on the regulatory γ subunit and this differential localisation may direct substrate selection and specify particular functional roles.
Figures
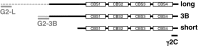




References
-
- Alzamora R, Gong F, Rondanino C, Lee JK, Smolak C, Pastor-Soler NM, Hallows KR. AMP-activated protein kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4-2 in renal epithelial cells. Am J Physiol Renal Physiol. 2010;299(6):F1308–F1319. doi: 10.1152/ajprenal.00423.2010. - DOI - PMC - PubMed
-
- Andersson L. Identification and characterization of AMPK gamma 3 mutations in the pig. Biochem Soc Trans. 2003;31(Pt 1):232–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

