a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III
- PMID: 24037882
- PMCID: PMC3816265
- DOI: 10.1210/jc.2013-2428
a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III
Abstract
Context: Primary aldosteronism is a heterogeneous group of disorders comprising both sporadic and familial forms. Mutations in the KCNJ5 gene, which encodes the inward rectifier K(+) channel 4 (G protein-activated inward rectifier K(+) channel 4, Kir3.4), cause familial hyperaldosteronism type III (FH-III) and are involved in the pathogenesis of sporadic aldosterone-producing adenomas.
Objective: The objective of the study was to characterize the effects of a newly described KCNJ5 mutation in vitro.
Patients and methods: The index case is a 62-year-old woman affected by primary aldosteronism, who underwent left adrenalectomy after workup for adrenal adenoma. Exon 1 of KCNJ5 was PCR amplified from adrenal tissue and peripheral blood and sequenced. Electrophysiological and gene expression studies were performed to establish the functional effects of the new mutation on the membrane potential and adrenal cell CYP11B2 expression.
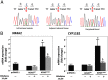
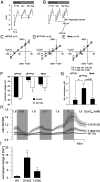
Results: KCNJ5 sequencing in the index case revealed a new p.Y152C germline mutation; interestingly, the phenotype of the patient was milder than most of the previously described FH-III families. The tyrosine-to-cysteine substitution resulted in pathological Na(+) permeability, cell membrane depolarization, and disturbed intracellular Ca(2+) homeostasis, effects similar, albeit smaller, to the ones demonstrated for other KCNJ5 mutations. Gene expression studies revealed an increased expression of CYP11B2 and its transcriptional regulator NR4A2 in HAC15 adrenal cells overexpressing KCNJ5(Y152C) compared to the wild-type channel. The effect was clearly Ca(2+)-dependent, because it was abolished by the calcium channel blocker nifedipine.
Conclusions: Herein we describe a new germline mutation in KCNJ5 responsible for FH-III.
Figures
References
-
- Mulatero P, Monticone S, Rainey WE, Veglio F, Williams TA. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol. 2013;9:104–112 - PubMed
-
- Mulatero P, Tauber P, Zennaro MC, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012;59:235–240 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

