All-trans-retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade
- PMID: 24038081
- PMCID: PMC4008145
- DOI: 10.1002/hep.26699
All-trans-retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade
Abstract
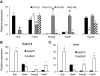
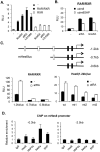
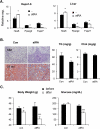
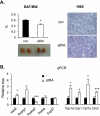
Mice deficient in small heterodimer partner (SHP) are protected from diet-induced hepatic steatosis resulting from increased fatty acid oxidation and decreased lipogenesis. The decreased lipogenesis appears to be a direct consequence of very low expression of peroxisome proliferator-activated receptor gamma 2 (PPAR-γ2), a potent lipogenic transcription factor, in the SHP(-/-) liver. The current study focused on the identification of a SHP-dependent regulatory cascade that controls PPAR-γ2 gene expression, thereby regulating hepatic fat accumulation. Illumina BeadChip array (Illumina, Inc., San Diego, CA) and real-time polymerase chain reaction were used to identify genes responsible for the linkage between SHP and PPAR-γ2 using hepatic RNAs isolated from SHP(-/-) and SHP-overexpressing mice. The initial efforts identify that hairy and enhancer of split 6 (Hes6), a novel transcriptional repressor, is an important mediator of the regulation of PPAR-γ2 transcription by SHP. The Hes6 promoter is specifically activated by the retinoic acid receptor (RAR) in response to its natural agonist ligand, all-trans retinoic acid (atRA), and is repressed by SHP. Hes6 subsequently represses hepatocyte nuclear factor 4 alpha (HNF-4α)-activated PPAR-γ2 gene expression by direct inhibition of HNF-4α transcriptional activity. Furthermore, we provide evidences that atRA treatment or adenovirus-mediated RAR-α overexpression significantly reduced hepatic fat accumulation in obese mouse models, as observed in earlier studies, and the beneficial effect is achieved by the proposed transcriptional cascade.
Conclusions: Our study describes a novel transcriptional regulatory cascade controlling hepatic lipid metabolism that identifies retinoic acid signaling as a new therapeutic approach to nonalcoholic fatty liver diseases.
© 2014 by the American Association for the Study of Liver Diseases.
Figures

Comment in
-
The hunt for treatment options of fatty liver continues: effects of retinoic acid on hepatic steatosis reveal novel transcriptional interactions of nuclear receptors.Hepatology. 2014 May;59(5):1662-4. doi: 10.1002/hep.26896. Epub 2014 Mar 27. Hepatology. 2014. PMID: 24122898 No abstract available.
Similar articles
-
Deletion of steroid receptor coactivator-3 gene ameliorates hepatic steatosis.J Hepatol. 2011 Aug;55(2):445-52. doi: 10.1016/j.jhep.2010.11.022. Epub 2010 Dec 22. J Hepatol. 2011. PMID: 21184786
-
The hunt for treatment options of fatty liver continues: effects of retinoic acid on hepatic steatosis reveal novel transcriptional interactions of nuclear receptors.Hepatology. 2014 May;59(5):1662-4. doi: 10.1002/hep.26896. Epub 2014 Mar 27. Hepatology. 2014. PMID: 24122898 No abstract available.
-
Disruption of hepatic small heterodimer partner induces dissociation of steatosis and inflammation in experimental nonalcoholic steatohepatitis.J Biol Chem. 2020 Jan 24;295(4):994-1008. doi: 10.1074/jbc.RA119.010233. Epub 2019 Dec 12. J Biol Chem. 2020. PMID: 31831621 Free PMC article.
-
Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones.J Endocrinol. 2017 Jul;234(1):R1-R21. doi: 10.1530/JOE-16-0513. Epub 2017 Apr 20. J Endocrinol. 2017. PMID: 28428362 Review.
-
Nonalcoholic fatty liver disease: molecular pathways and therapeutic strategies.Lipids Health Dis. 2013 Nov 9;12:171. doi: 10.1186/1476-511X-12-171. Lipids Health Dis. 2013. PMID: 24209497 Free PMC article. Review.
Cited by
-
A retinoic acid receptor β2 agonist reduces hepatic stellate cell activation in nonalcoholic fatty liver disease.J Mol Med (Berl). 2016 Oct;94(10):1143-1151. doi: 10.1007/s00109-016-1434-z. Epub 2016 Jun 6. J Mol Med (Berl). 2016. PMID: 27271256 Free PMC article.
-
Alisol B Alleviates Hepatocyte Lipid Accumulation and Lipotoxicity via Regulating RARα-PPARγ-CD36 Cascade and Attenuates Non-Alcoholic Steatohepatitis in Mice.Nutrients. 2022 Jun 10;14(12):2411. doi: 10.3390/nu14122411. Nutrients. 2022. PMID: 35745142 Free PMC article.
-
All-Trans Retinoic Acid Inhibits Bone Marrow Mesenchymal Stem Cell Commitment to Adipocytes via Upregulating FRA1 Signaling.Int J Endocrinol. 2020 Jan 31;2020:6525787. doi: 10.1155/2020/6525787. eCollection 2020. Int J Endocrinol. 2020. PMID: 32089684 Free PMC article.
-
The role of the retinoid receptor, RAR/RXR heterodimer, in liver physiology.Biochim Biophys Acta Mol Basis Dis. 2021 May 1;1867(5):166085. doi: 10.1016/j.bbadis.2021.166085. Epub 2021 Jan 24. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33497820 Free PMC article. Review.
-
Retinoids in the Pathogenesis and Treatment of Liver Diseases.Nutrients. 2022 Mar 31;14(7):1456. doi: 10.3390/nu14071456. Nutrients. 2022. PMID: 35406069 Free PMC article. Review.
References
-
- Johansson L, Thomsen JS, Damdimopoulos AE, Spyrou G, Gustafsson J, Treuter E. The orphan nuclear receptor SHP inhibits agonist-dependent transcriptional activity of estrogen receptors ERalpha and ERbeta. J Biol Chem. 1999;274:345–353. - PubMed
-
- Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, et al. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet. 2001;27:375–382. - PubMed
-
- Hung CC, Farooqi IS, Ong K, Luan J, Keogh JM, Pembrey M, Yeo GS, et al. Contribution of variants in the small heterodimer partner gene to birthweight, adiposity, and insulin levels: mutational analysis and association studies in multiple populations. Diabetes. 2003;52:1288–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
