A comparison of calcium to zoledronic acid for improvement of cortical bone in an animal model of CKD
- PMID: 24038306
- PMCID: PMC3940692
- DOI: 10.1002/jbmr.2089
A comparison of calcium to zoledronic acid for improvement of cortical bone in an animal model of CKD
Abstract
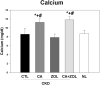
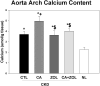
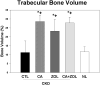
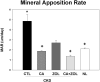
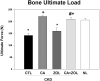
Patients with chronic kidney disease (CKD) have increased risk of fractures, yet the optimal treatment is unknown. In secondary analyses of large randomized trials, bisphosphonates have been shown to improve bone mineral density and reduce fractures. However, bisphosphonates are currently not recommended in patients with advanced kidney disease due to concern about oversuppressing bone remodeling, which may increase the risk of developing arterial calcification. In the present study we used a naturally occurring rat model of CKD with secondary hyperparathyroidism, the Cy/+ rat, and compared the efficacy of treatment with zoledronic acid, calcium given in water to simulate a phosphate binder, and the combination of calcium and zoledronic acid. Animals were treated beginning at 25 weeks of age (approximately 30% of normal renal function) and followed for 10 weeks. The results demonstrate that both zoledronic acid and calcium improved bone volume by micro-computed tomography (µCT) and both equally suppressed the mineral apposition rate, bone formation rate, and mineralizing surface of trabecular bone. In contrast, only calcium treatment with or without zoledronic acid improved cortical porosity and cortical biomechanical properties (ultimate load and stiffness) and lowered parathyroid hormone (PTH). However, only calcium treatment led to the adverse effects of increased arterial calcification and fibroblast growth factor 23 (FGF23). These results suggest zoledronic acid may improve trabecular bone volume in CKD in the presence of secondary hyperparathyroidism, but does not benefit extraskeletal calcification or cortical biomechanical properties. Calcium effectively reduces PTH and benefits both cortical and trabecular bone yet increases the degree of extra skeletal calcification. © 2014 American Society for Bone and Mineral Research.
Keywords: BIOMECHANICS; BONE; CALCIUM; CKD; FGF23; PARATHYROID HORMONE; VASCULAR CALCIFICATION; ZOLEDRONIC ACID.
© 2014 American Society for Bone and Mineral Research.
Conflict of interest statement
Figures







Similar articles
-
Skeletal effects of zoledronic acid in an animal model of chronic kidney disease.Osteoporos Int. 2013 Apr;24(4):1471-81. doi: 10.1007/s00198-012-2103-x. Epub 2012 Aug 21. Osteoporos Int. 2013. PMID: 22907737 Free PMC article.
-
Effects of unfractionated heparin on renal osteodystrophy and vascular calcification in chronic kidney disease rats.Bone. 2014 Jan;58:168-76. doi: 10.1016/j.bone.2013.10.010. Epub 2013 Oct 18. Bone. 2014. PMID: 24145307
-
Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy.J Bone Miner Res. 2015 Mar;30(3):499-509. doi: 10.1002/jbmr.2372. J Bone Miner Res. 2015. PMID: 25407607 Free PMC article.
-
Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment.Semin Oncol. 2001 Apr;28(2 Suppl 6):35-44. doi: 10.1016/s0093-7754(01)90263-5. Semin Oncol. 2001. PMID: 11346863 Review.
-
Zoledronic acid: a review of its use in patients with advanced cancer.Drugs. 2004;64(11):1197-211. doi: 10.2165/00003495-200464110-00004. Drugs. 2004. PMID: 15161327 Review.
Cited by
-
Intracellular calcium increases in vascular smooth muscle cells with progression of chronic kidney disease in a rat model.Nephrol Dial Transplant. 2017 Mar 1;32(3):450-458. doi: 10.1093/ndt/gfw274. Nephrol Dial Transplant. 2017. PMID: 27510531 Free PMC article.
-
Bisphosphonates and bone mineral density in patients with end-stage kidney disease and renal transplants: A 15-year single-centre experience.Bone Rep. 2022 Mar 4;16:101178. doi: 10.1016/j.bonr.2022.101178. eCollection 2022 Jun. Bone Rep. 2022. PMID: 35281213 Free PMC article.
-
A microRNA Approach to Discriminate Cortical Low Bone Turnover in Renal Osteodystrophy.JBMR Plus. 2020 Mar 25;4(5):e10353. doi: 10.1002/jbm4.10353. eCollection 2020 May. JBMR Plus. 2020. PMID: 32490328 Free PMC article.
-
Changes in skeletal collagen cross-links and matrix hydration in high- and low-turnover chronic kidney disease.Osteoporos Int. 2015 Mar;26(3):977-85. doi: 10.1007/s00198-014-2978-9. Epub 2014 Dec 3. Osteoporos Int. 2015. PMID: 25466530 Free PMC article.
-
Skeletal vascular perfusion is altered in chronic kidney disease.Bone Rep. 2018 May 4;8:215-220. doi: 10.1016/j.bonr.2018.05.001. eCollection 2018 Jun. Bone Rep. 2018. PMID: 29955640 Free PMC article.
References
-
- Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–53. - PubMed
-
- Lindberg JS, Moe SM. Osteoporosis in end-state renal disease. Semin Nephrol. 1999;19(2):115–22. - PubMed
-
- Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20(12):2105–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

