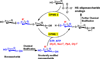
Tailored design and synthesis of heparan sulfate oligosaccharide analogues using sequential one-pot multienzyme systems
- PMID: 24038939
- PMCID: PMC3943747
- DOI: 10.1002/anie.201305667
Tailored design and synthesis of heparan sulfate oligosaccharide analogues using sequential one-pot multienzyme systems
Keywords: carbohydrates; chemoenzymatic systems; enzymes; heparin; structure-activity relationships.
Figures
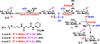
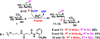



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

