Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy
- PMID: 24038954
- PMCID: PMC3946335
- DOI: 10.1002/smll.201301456
Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy
Abstract
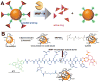
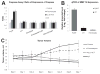
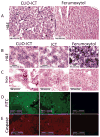
A major drawback with current cancer therapy is the prevalence of unrequired dose-limiting toxicity to non-cancerous tissues and organs, which is further compounded by a limited ability to rapidly and easily monitor drug delivery, pharmacodynamics and therapeutic response. In this report, the design and characterization of novel multifunctional "theranostic" nanoparticles (TNPs) is described for enzyme-specific drug activation at tumor sites and simultaneous in vivo magnetic resonance imaging (MRI) of drug delivery. TNPs are synthesized by conjugation of FDA-approved iron oxide nanoparticles ferumoxytol to an MMP-activatable peptide conjugate of azademethylcolchicine (ICT), creating CLIO-ICTs (TNPs). Significant cell death is observed in TNP-treated MMP-14 positive MMTV-PyMT breast cancer cells in vitro, but not MMP-14 negative fibroblasts or cells treated with ferumoxytol alone. Intravenous administration of TNPs to MMTV-PyMT tumor-bearing mice and subsequent MRI demonstrates significant tumor selective accumulation of the TNP, an observation confirmed by histopathology. Treatment with CLIO-ICTs induces a significant antitumor effect and tumor necrosis, a response not observed with ferumoxytol. Furthermore, no toxicity or cell death is observed in normal tissues following treatment with CLIO-ICTs, ICT, or ferumoxytol. These findings demonstrate proof of concept for a new nanotemplate that integrates tumor specificity, drug delivery and in vivo imaging into a single TNP entity through attachment of enzyme-activated prodrugs onto magnetic nanoparticles. This novel approach holds the potential to significantly improve targeted cancer therapies, and ultimately enable personalized therapy regimens.
Keywords: MMP-14; MR imaging; cancer therapy; iron oxide; nanoparticles; theranostic.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Mura S, Couvreur P. Adv Drug Delivery Rev. 2012;64:1394–1416. - PubMed
- Jain RK, Stylianopoulos T. Nat Rev Clin Oncol. 2010;7:653–664. - PMC - PubMed
- Lammers T, Kiessling F, Hennink WE, Storm G. Journal of Control Release. 2012;161:175–187. - PubMed
- Terreno E, Uggeri F, Aime S. Journal of Control Release. 2012;161:328–337. - PubMed
-
- Greish K. In: Cancer Nanotechnology: Methods and Protocols. Grobmyer M, editor. Vol. 624. 2010. pp. 25–37.
-
- Marcucci F, Lefoulon F. Drug Discov Today. 2004;9:219–228. - PubMed
-
- Reyes-Aldasoro CC, Wilson I, Prise VE, Barber PR, Ameer-Beg SM, Vojnovic B, Cunningham VJ, Tozer GM. Microcirculation. 2008;15:65–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 CA138353/CA/NCI NIH HHS/United States
- P50CA114747/CA/NCI NIH HHS/United States
- R01 CA170378/CA/NCI NIH HHS/United States
- R21 CA156124/CA/NCI NIH HHS/United States
- R01CA170378/CA/NCI NIH HHS/United States
- R01CA140943/CA/NCI NIH HHS/United States
- R01 CA140943/CA/NCI NIH HHS/United States
- R01 CA105102/CA/NCI NIH HHS/United States
- P30 CA124435/CA/NCI NIH HHS/United States
- P50 CA114747/CA/NCI NIH HHS/United States
- U54 CA151459/CA/NCI NIH HHS/United States
- R01CA105102/CA/NCI NIH HHS/United States
- R01 CA135294/CA/NCI NIH HHS/United States
- R21CA156124/CA/NCI NIH HHS/United States
- P30CA124435-02/CA/NCI NIH HHS/United States
- R01CA135294/CA/NCI NIH HHS/United States
- U54CA151459/CA/NCI NIH HHS/United States
- R21CA138353A2/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

