Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential
- PMID: 24039004
- PMCID: PMC4001734
- DOI: 10.1002/smll.201301597
Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential
Abstract
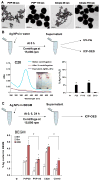
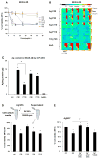
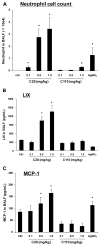
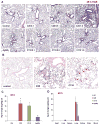
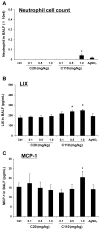
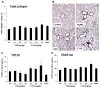
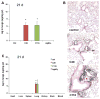
Since more than 30% of consumer products that include engineered nanomaterials contain nano-Ag, the safety of this material is of considerable public concern. In this study, Ag nanoparticles (NPs) are used to demonstrate that 20 nm polyvinylpyrrolidone (PVP or P) and citrate (C)-coated Ag NPs induce more cellular toxicity and oxidative stress than larger (110 nm) particles due to a higher rate of dissolution and Ag bioavailability. Moreover, there is also a higher propensity for citrate 20 nm (C20) nanoparticles to generate acute neutrophilic inflammation in the lung and to produce chemokines compared to C110. P110 has less cytotoxic effects than C110, likely due to the ability of PVP to complex released Ag(+) . In contrast to the more intense acute pulmonary effects of C20, C110 induces mild pulmonary fibrosis at day 21, likely as a result of slow but persistent Ag(+) release leading to a sub-chronic injury response. Interestingly, the released metallic Ag is incorporated into the collagen fibers depositing around airways and the lung interstitium. Taken together, these results demonstrate that size and surface coating affect the cellular toxicity of Ag NPs as well as their acute versus sub-chronic lung injury potential.
Keywords: cytotoxicity; dissolution; lung inflammation; nanoparticle; silver.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van de Meent D, Dekkers S, De Jong WH, Van Zijverden M, Sips AJAM, Geertsma RE. Nanotoxicology. 2009;3:109–U178.
-
- Glover RD, Miller JM, Hutchison JE. Acs Nano. 2011;5:8950–8957. - PubMed
-
- Kim WY, Kim J, Park JD, Ryu HY, Yu IJ. J Toxicol Environ Health Part A. 2009;72:1279–1284. - PubMed
-
- Sung JH, Ji JH, Yoon JU, Kim DS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Kim J, Kim TS, Chang HK, Lee EJ, Lee JH, Yu IJ. Inhal Toxicol. 2008;20:567–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

