Estimation of Progression-Free Survival for All Treated Patients in the Randomized Discontinuation Trial Design
- PMID: 24039273
- PMCID: PMC3769804
- DOI: 10.1080/00031305.2012.720900
Estimation of Progression-Free Survival for All Treated Patients in the Randomized Discontinuation Trial Design
Abstract
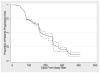
The randomized discontinuation trial (RDT) design is an enrichment-type design that has been used in a variety of diseases to evaluate the efficacy of new treatments. The RDT design seeks to select a more homogeneous group of patients, consisting of those who are more likely to show a treatment benefit if one exists. In oncology, the RDT design has been applied to evaluate the effects of cytostatic agents, that is, drugs that act primarily by slowing tumor growth rather than shrinking tumors. In the RDT design, all patients receive treatment during an initial, open-label run-in period of duration T. Patients with objective response (substantial tumor shrinkage) remain on therapy while those with early progressive disease are removed from the trial. Patients with stable disease (SD) are then randomized to either continue active treatment or switched to placebo. The main analysis compares outcomes, for example, progression-free survival (PFS), between the two randomized arms. As a secondary objective, investigators may seek to estimate PFS for all treated patients, measured from the time of entry into the study, by combining information from the run-in and post run-in periods. For t ≤ T, PFS is estimated by the observed proportion of patients who are progression-free among all patients enrolled. For t > T, the estimate can be expressed as Ŝ(t) = p̂OR × ŜOR(t - T) + p̂SD × ŜSD(t - T), where p̂OR is the estimated probability of response during the run-in period, p̂SD is the estimated probability of SD, and ŜOR(t - T) and ŜSD(t - T) are the Kaplan-Meier estimates of subsequent PFS in the responders and patients with SD randomized to continue treatment, respectively. In this article, we derive the variance of Ŝ(t), enabling the construction of confidence intervals for both S(t) and the median survival time. Simulation results indicate that the method provides accurate coverage rates. An interesting aspect of the design is that outcomes during the run-in phase have a negative multinomial distribution, something not frequently encountered in practice.
Keywords: Confidence limits; Enrichment design; Negative multinomial distribution; Phase II clinical trials.
Figures


References
-
- Bishop YMM, Fienberg SE, Holland PW. Discrete Multivariate Analysis. Cambridge: MIT Press; 1975.
-
- Brookmeyer R, Crowley J. A Confidence Interval for the Median Survival Time. Biometrics. 1982;38:29–41.
-
- Capra W. Comparing the Power of the Discontinuation Design to That of the Classic Randomized Design on Time-to-Event Endpoints. Controlled Clinical Trials. 2004;25:168–177. - PubMed
-
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM TARGET Study Group. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. New England Journal of Medicine. 2007;356:125–134. - PubMed
-
- Freidlin B, Simon R. Evaluation of Randomized Discontinuation Design. Journal of Clinical Oncology. 2005;23:5094–5098. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
