Multicellular bacteria deploy the type VI secretion system to preemptively strike neighboring cells
- PMID: 24039579
- PMCID: PMC3764213
- DOI: 10.1371/journal.ppat.1003608
Multicellular bacteria deploy the type VI secretion system to preemptively strike neighboring cells
Abstract
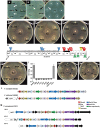
The Type VI Secretion System (T6SS) functions in bacteria as a contractile nanomachine that punctures and delivers lethal effectors to a target cell. Virtually nothing is known about the lifestyle or physiology that dictates when bacteria normally produce their T6SS, which prevents a clear understanding of how bacteria benefit from its action in their natural habitat. Proteus mirabilis undergoes a characteristic developmental process to coordinate a multicellular swarming behavior and will discriminate itself from another Proteus isolate during swarming, resulting in a visible boundary termed a Dienes line. Using transposon mutagenesis, we discovered that this recognition phenomenon requires the lethal action of the T6SS. All mutants identified in the genetic screen had insertions within a single 33.5-kb region that encodes a T6SS and cognate Hcp-VrgG-linked effectors. The identified T6SS and primary effector operons were characterized by killing assays, by construction of additional mutants, by complementation, and by examining the activity of the type VI secretion system in real-time using live-cell microscopy on opposing swarms. We show that lethal T6SS-dependent activity occurs when a dominant strain infiltrates deeply beyond the boundary of the two swarms. Using this multicellular model, we found that social recognition in bacteria, underlying killing, and immunity to killing all require cell-cell contact, can be assigned to specific genes, and are dependent on the T6SS. The ability to survive a lethal T6SS attack equates to "recognition". In contrast to the current model of T6SS being an offensive or defensive weapon our findings support a preemptive mechanism by which an entire population indiscriminately uses the T6SS for contact-dependent delivery of effectors during its cooperative mode of growth.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

