Bi-modal distribution of the second messenger c-di-GMP controls cell fate and asymmetry during the caulobacter cell cycle
- PMID: 24039597
- PMCID: PMC3764195
- DOI: 10.1371/journal.pgen.1003744
Bi-modal distribution of the second messenger c-di-GMP controls cell fate and asymmetry during the caulobacter cell cycle
Abstract
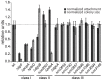
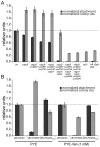
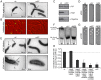
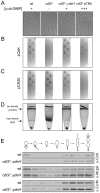
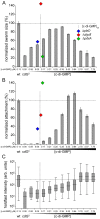
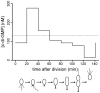
Many bacteria mediate important life-style decisions by varying levels of the second messenger c-di-GMP. Behavioral transitions result from the coordination of complex cellular processes such as motility, surface adherence or the production of virulence factors and toxins. While the regulatory mechanisms responsible for these processes have been elucidated in some cases, the global pleiotropic effects of c-di-GMP are poorly understood, primarily because c-di-GMP networks are inherently complex in most bacteria. Moreover, the quantitative relationships between cellular c-di-GMP levels and c-di-GMP dependent phenotypes are largely unknown. Here, we dissect the c-di-GMP network of Caulobacter crescentus to establish a global and quantitative view of c-di-GMP dependent processes in this organism. A genetic approach that gradually reduced the number of diguanylate cyclases identified novel c-di-GMP dependent cellular processes and unraveled c-di-GMP as an essential component of C. crescentus cell polarity and its bimodal life cycle. By varying cellular c-di-GMP concentrations, we determined dose response curves for individual c-di-GMP-dependent processes. Relating these values to c-di-GMP levels modeled for single cells progressing through the cell cycle sets a quantitative frame for the successive activation of c-di-GMP dependent processes during the C. crescentus life cycle. By reconstructing a simplified c-di-GMP network in a strain devoid of c-di-GMP we defined the minimal requirements for the oscillation of c-di-GMP levels during the C. crescentus cell cycle. Finally, we show that although all c-di-GMP dependent cellular processes were qualitatively restored by artificially adjusting c-di-GMP levels with a heterologous diguanylate cyclase, much higher levels of the second messenger are required under these conditions as compared to the contribution of homologous c-di-GMP metabolizing enzymes. These experiments suggest that a common c-di-GMP pool cannot fully explain spatiotemporal regulation by c-di-GMP in C. crescentus and that individual enzymes preferentially regulate specific phenotypes during the cell cycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Jenal U, Malone J (2006) Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet 40: 385–407 doi:10.1146/annurev.genet.40.110405.090423 - DOI - PubMed
-
- Hengge R (2009) Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7: 263–273 doi:10.1038/nrmicro2109 - DOI - PubMed
-
- Galperin MY, Nikolskaya AN, Koonin EV (2001) Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett 203: 11–21. - PubMed
-
- Sondermann H, Shikuma NJ, Yildiz FH (2012) You've come a long way: c-di-GMP signaling. Curr Opin Microbiol 15: 140–146 doi:10.1016/j.mib.2011.12.008 - DOI - PMC - PubMed
-
- Pultz IS, Christen M, Kulasekara HD, Kennard A, Kulasekara B, et al. (2012) The response threshold of Salmonella PilZ domain proteins is determined by their binding affinities for c-di-GMP. Mol Microbiol 86: 1424–1440 doi:10.1111/mmi.12066 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

