Protein kinase C beta mediates CD40 ligand-induced adhesion of monocytes to endothelial cells
- PMID: 24039784
- PMCID: PMC3767684
- DOI: 10.1371/journal.pone.0072593
Protein kinase C beta mediates CD40 ligand-induced adhesion of monocytes to endothelial cells
Retraction in
-
Retraction: Protein Kinase C beta Mediates CD40 Ligand-Induced Adhesion of Monocytes to Endothelial Cells.PLoS One. 2023 Dec 5;18(12):e0295636. doi: 10.1371/journal.pone.0295636. eCollection 2023. PLoS One. 2023. PMID: 38051709 Free PMC article. No abstract available.
Abstract
Accumulating evidence supports the early involvement of monocyte/macrophage recruitment to activated endothelial cells by leukocyte adhesion molecules during atherogenesis. CD40 and its ligand CD40L are highly expressed in vascular endothelial cells, but its impact on monocyte adhesion and the related molecular mechanisms are not fully understood. The present study was designed to evaluate the direct effect of CD40L on monocytic cell adhesion and gain mechanistic insight into the signaling coupling CD40L function to the proinflammatory response. Exposure of cultured human aortic endothelial cells (HAECs) to clinically relevant concentrations of CD40L (20 to 80 ng/mL) dose-dependently increased human monocytic THP-1 cells to adhere to them under static condition. CD40L treatment induced the expression of vascular cell adhesion molecule-1 (VCAM-1) mRNA and protein expression in HAECs. Furthermore, exposure of HAECs to CD40L robustly increased the activation of protein kinase C beta (PKCβ) in ECs. A selective inhibitor of PKCβ prevented the rise in VCAM-1 and THP-1 cell adhesion to ECs. Moreover, stimulation of ECs to CD40L induced nuclear factor-κB (NF-κB) activation. PKCβ inhibition abolished CD40L-induced NF-κB activation, and NF-κB inhibition reduced expression of VCAM-1, each resulting in reduced THP-1 cell adhesion. Our findings provide the evidence that CD40L increases VCAM-1 expression in ECs by activating PKCβ and NF-κB, suggesting a novel mechanism for EC activation. Finally, administration of CD40L resulted in PKCβ activation, increased VCAM-1 expression and activated monocytes adhesiveness to HAECs, processes attenuated by PKCβ inhibitor. Therefore, CD40L may contribute directly to atherogenesis by activating ECs and recruiting monocytes to them.
Conflict of interest statement
Figures







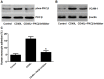
References
-
- Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340: 115–126. - PubMed
-
- Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6: 508–519. - PubMed
-
- Galkina E, Ley K (2007) Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2292–2301. - PubMed
-
- Kawakami A, Tanaka A, Nakajima K, Shimokado K, Yoshida M (2002) Atorvastatin attenuates remnant lipoprotein-induced monocyte adhesion to vascular endothelium under flow conditions. Circ Res 91: 263–271. - PubMed
-
- Schonbeck U, Lippy P (2001) CD40 signaling and plaque instability. Circ Res 89: 1092–1103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

