Paraquat poisoning induces TNF-α-dependent iNOS/NO mediated hyporesponsiveness of the aorta to vasoconstrictors in rats
- PMID: 24039983
- PMCID: PMC3767802
- DOI: 10.1371/journal.pone.0073562
Paraquat poisoning induces TNF-α-dependent iNOS/NO mediated hyporesponsiveness of the aorta to vasoconstrictors in rats
Abstract
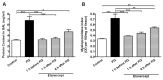
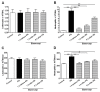
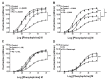
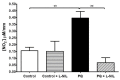
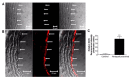
Paraquat is a toxic herbicide that may induce acute lung injury, circulatory failure and death. The present work aimed at investigating whether there is systemic inflammation and vascular dysfunction after paraquat exposure and whether these parameters were related. There was neutrophilia and accumulation of neutrophils in lung and bronchoalveolar lavage of animals given paraquat. This was associated with an increase in serum levels of TNF-α. In rats given paraquat, the relaxant response of aortic rings to acetylcholine was not modified but the contractile response to phenylephrine was greatly reduced. Endothelium removal or treatment with non-selective (L-NAME) or selective (L-NIL) inhibitors of inducible nitric oxide synthase (iNOS) restored contraction of aortas. There was greater production of nitric oxide (NO), which was restored to basal level by L-NIL, and greater expression of iNOS in endothelial cells, as seen by Western blot analyses and confocal microscopy. Blockade of TNF-α reduced pulmonary and systemic inflammation and vascular dysfunction. Together, our results clearly show that paraquat causes pulmonary and systemic inflammation, and vascular dysfunction in rats. Vascular dysfunction is TNF-α dependent, associated with enhanced expression of iNOS in aortic endothelial cells and greater NO production, which accounts for the decreased responsiveness of aortas to vasoconstrictors. Blockers of TNF-α may be useful in patients with paraquat poisoning.
Conflict of interest statement
Figures




References
-
- Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML et al. (2008) Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 38: 13-71. doi:10.1080/10408440701669959. PubMed: 18161502. - DOI - PubMed
-
- Mainwaring G, Lim FL, Antrobus K, Swain C, Clapp M et al. (2006) Identification of early molecular pathways affected by paraquat in rat lung. Toxicology 225: 157-172. doi:10.1016/j.tox.2006.05.017. PubMed: 16854511. - DOI - PubMed
-
- Rose MS, Lock EA, Smith LL, Wyatt I (1976) Paraquat accumulation: tissue and species specificity. Biochem Pharmacol 25: 419-423. doi:10.1016/0006-2952(76)90344-0. PubMed: 820354. - DOI - PubMed
-
- Rose MS, Smith LL, Wyatt I (1974) Evidence for Energy-Dependent Accumulation of Paraquat into Rat Lung. Nature 252: 314-315. doi:10.1038/252314a0. PubMed: 4431454. - DOI - PubMed
-
- Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS (1999) A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A 96: 12760-12765. doi:10.1073/pnas.96.22.12760. PubMed: 10535996. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

