Muricholic acids inhibit Clostridium difficile spore germination and growth
- PMID: 24040011
- PMCID: PMC3767737
- DOI: 10.1371/journal.pone.0073653
Muricholic acids inhibit Clostridium difficile spore germination and growth
Abstract
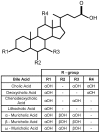
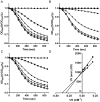
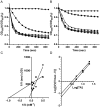
Infections caused by Clostridium difficile have increased steadily over the past several years. While studies on C. difficile virulence and physiology have been hindered, in the past, by lack of genetic approaches and suitable animal models, newly developed technologies and animal models allow these processes to be studied in detail. One such advance is the generation of a mouse-model of C. difficile infection. The development of this system is a major step forward in analyzing the genetic requirements for colonization and infection. While important, it is equally as important in understanding what differences exist between mice and humans. One of these differences is the natural bile acid composition. Bile acid-mediated spore germination is an important step in C. difficile colonization. Mice produce several different bile acids that are not found in humans. These muricholic acids have the potential to impact C. difficile spore germination. Here we find that the three muricholic acids (α-muricholic acid, β-muricholic acid and ω-muricholic acid) inhibit C. difficile spore germination and can impact the growth of vegetative cells. These results highlight an important difference between humans and mice and may have an impact on C. difficile virulence in the mouse-model of C. difficile infection.
Conflict of interest statement
Figures
References
-
- Kyne L, Hamel MB, Polavaram R, Kelly CP (2002) Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile . Clin Infect Dis 34: 346–353. - PubMed
-
- O’Brien JA, Lahue BJ, Caro JJ, Davidson DM (2007) The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 28: 1219–1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

