Statins decrease lung inflammation in mice by upregulating tetraspanin CD9 in macrophages
- PMID: 24040034
- PMCID: PMC3767596
- DOI: 10.1371/journal.pone.0073706
Statins decrease lung inflammation in mice by upregulating tetraspanin CD9 in macrophages
Abstract
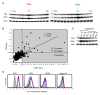
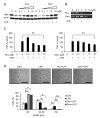
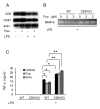
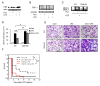
Tetraspanins organize protein complexes in tetraspanin-enriched membrane microdomains that are distinct from lipid rafts. Our previous studies suggested that reduction in the levels of tetraspanins CD9 and CD81 may be involved in the progression of inflammatory lung diseases, especially COPD. To search for agents that increase the levels of these tetraspanins, we screened 1,165 drugs in clinical use and found that statins upregulate CD9 and CD81 in RAW264.7 macrophages. The lipophilic statins, fluvastatin and simvastatin, reversed LPS-induced downregulation of CD9 and CD81, simultaneously preventing TNF-α and matrix metalloproteinase-9 production and spreading of RAW264.7 cells. These statins exerted anti-inflammatory effects in vitro in wild-type macrophages but not in CD9 knockout macrophages, and decreased lung inflammation in vivo in wild-type mice but not in CD9 knockout mice, suggesting that their effects are dependent on CD9. Mechanistically, the statins promoted reverse transfer of the LPS-signaling mediator CD14 from lipid rafts into CD9-enriched microdomains, thereby preventing LPS receptor formation. Finally, upregulation of CD9/CD81 by statins was related to blockade of GTPase geranylgeranylation in the mevalonate pathway. Our data underscore the importance of the negative regulator CD9 in lung inflammation, and suggest that statins exert anti-inflammatory effects by upregulating tetraspanin CD9 in macrophages.
Conflict of interest statement
Figures


Similar articles
-
Tetraspanin CD9 negatively regulates lipopolysaccharide-induced macrophage activation and lung inflammation.J Immunol. 2009 May 15;182(10):6485-93. doi: 10.4049/jimmunol.0802797. J Immunol. 2009. PMID: 19414803
-
A role for tetraspanin proteins in regulating fusion induced by Burkholderia thailandensis.Med Microbiol Immunol. 2020 Aug;209(4):473-487. doi: 10.1007/s00430-020-00670-6. Epub 2020 Apr 6. Med Microbiol Immunol. 2020. PMID: 32253503 Free PMC article.
-
Deletion of tetraspanin CD9 diminishes lymphangiogenesis in vivo and in vitro.J Biol Chem. 2013 Jan 25;288(4):2118-31. doi: 10.1074/jbc.M112.424291. Epub 2012 Dec 5. J Biol Chem. 2013. PMID: 23223239 Free PMC article.
-
Preventive Role of Tetraspanin CD9 in Systemic Inflammation of Chronic Obstructive Pulmonary Disease.Am J Respir Cell Mol Biol. 2015 Dec;53(6):751-60. doi: 10.1165/rcmb.2015-0122TR. Am J Respir Cell Mol Biol. 2015. PMID: 26378766 Review.
-
Viruses and tetraspanins: lessons from single molecule approaches.Viruses. 2014 May 5;6(5):1992-2011. doi: 10.3390/v6051992. Viruses. 2014. PMID: 24800676 Free PMC article. Review.
Cited by
-
Synergistic protective effects of a statin and an angiotensin receptor blocker for initiation and progression of atherosclerosis.PLoS One. 2019 May 3;14(5):e0215604. doi: 10.1371/journal.pone.0215604. eCollection 2019. PLoS One. 2019. PMID: 31050669 Free PMC article.
-
Tetraspanins set the stage for bone marrow microenvironment-induced chemoprotection in hematologic malignancies.Blood Adv. 2023 Aug 22;7(16):4403-4413. doi: 10.1182/bloodadvances.2023010476. Blood Adv. 2023. PMID: 37561544 Free PMC article. Review.
-
Proteomic change in the upper lobe of the left lung of Beagle dogs at the lung migration stage of Toxocara canis infection.Parasit Vectors. 2024 May 9;17(1):210. doi: 10.1186/s13071-024-06302-9. Parasit Vectors. 2024. PMID: 38725025 Free PMC article.
-
Roles of the Mevalonate Pathway and Cholesterol Trafficking in Pulmonary Host Defense.Curr Mol Pharmacol. 2017;10(1):27-45. doi: 10.2174/1874467209666160112123603. Curr Mol Pharmacol. 2017. PMID: 26758950 Free PMC article.
-
Dose-response relationships in gene expression profiles in a harbor seal B lymphoma cell line exposed to 17 -ethinyl estradiol.J Xenobiot. 2017 May 23;7(1):6702. doi: 10.4081/xeno.2017.6702. eCollection 2017 Apr 28. J Xenobiot. 2017. PMID: 30701058 Free PMC article. No abstract available.
References
-
- Barnes PJ (2009) Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol 71: 451-464. doi:10.1146/annurev.physiol.010908.163257. PubMed: 18817512. - DOI - PubMed
-
- Fabbri LM, Rabe KF (2007) From COPD to chronic systemic inflammatory syndrome? Lancet 370: 797-799. doi:10.1016/S0140-6736(07)61383-X. PubMed: 17765529. - DOI - PubMed
-
- Barnes PJ, Celli BR (2009) Systemic manifestations and comorbidities of COPD. Eur Respir J 33: 1165-1185. doi:10.1183/09031936.00128008. PubMed: 19407051. - DOI - PubMed
-
- Fabbri LM, Luppi F, Beghé B, Rabe KF (2008) Complex chronic comorbidities of COPD. Eur Respir J 31: 204-212. doi:10.1183/09031936.00114307. PubMed: 18166598. - DOI - PubMed
-
- Clowes JA, Riggs BL, Khosla S (2005) The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208: 207-227. doi:10.1111/j.0105-2896.2005.00334.x. PubMed: 16313351. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

