Cold atmospheric plasma for selectively ablating metastatic breast cancer cells
- PMID: 24040051
- PMCID: PMC3770688
- DOI: 10.1371/journal.pone.0073741
Cold atmospheric plasma for selectively ablating metastatic breast cancer cells
Abstract
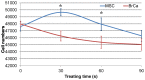
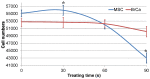
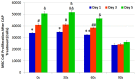
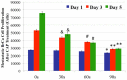
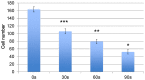
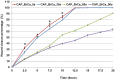
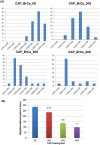
Traditional breast cancer treatments such as surgery and radiotherapy contain many inherent limitations with regards to incomplete and nonselective tumor ablation. Cold atmospheric plasma (CAP) is an ionized gas where the ion temperature is close to room temperature. It contains electrons, charged particles, radicals, various excited molecules, UV photons and transient electric fields. These various compositional elements have the potential to either enhance and promote cellular activity, or disrupt and destroy them. In particular, based on this unique composition, CAP could offer a minimally-invasive surgical approach allowing for specific cancer cell or tumor tissue removal without influencing healthy cells. Thus, the objective of this research is to investigate a novel CAP-based therapy for selectively bone metastatic breast cancer treatment. For this purpose, human metastatic breast cancer (BrCa) cells and bone marrow derived human mesenchymal stem cells (MSCs) were separately treated with CAP, and behavioral changes were evaluated after 1, 3, and 5 days of culture. With different treatment times, different BrCa and MSC cell responses were observed. Our results showed that BrCa cells were more sensitive to these CAP treatments than MSCs under plasma dose conditions tested. It demonstrated that CAP can selectively ablate metastatic BrCa cells in vitro without damaging healthy MSCs at the metastatic bone site. In addition, our study showed that CAP treatment can significantly inhibit the migration and invasion of BrCa cells. The results suggest the great potential of CAP for breast cancer therapy.
Conflict of interest statement
Figures







References
-
- Fridman A (2008) Plasma Chemistry. Cambridge: Cambridge University Press. 1017 p.
-
- Cooper M, Fridman G, Staack D, Gutsol A, Vasilets V, et al. (2009) Decontamination of Surfaces From Extremophile Organisms Using Nonthermal Atmospheric-Pressure Plasmas. IEEE Trans Plasma Sci 37: 866–871.
-
- Feng H, Wang R, Sun P, Wu H, Liu Q, et al... (2010) Atmospheric pressure cold plasma as an antifungal therapy. Appl Phys Lett 97.
-
- Shimizu T, Steffes B, Pompl R, Jamitzky F, Bunk W (2008) Characterization of microwave plasma torch for decontamination. Plasma Processes and Polymers 5: 577–582.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

