Stability of the acetic acid-induced bladder irritation model in alpha chloralose-anesthetized female cats
- PMID: 24040064
- PMCID: PMC3767621
- DOI: 10.1371/journal.pone.0073771
Stability of the acetic acid-induced bladder irritation model in alpha chloralose-anesthetized female cats
Abstract
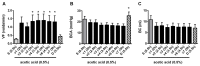
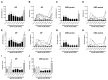
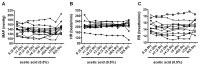
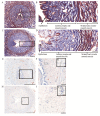
Time- and vehicle-related variability of bladder and urethral rhabdosphincter (URS) activity as well as cardiorespiratory and blood chemistry values were examined in the acetic acid-induced bladder irritation model in α-chloralose-anesthetized female cats. Additionally, bladder and urethra were evaluated histologically using Mason trichrome and toluidine blue staining. Urodynamic, cardiovascular and respiratory parameters were collected during intravesical saline infusion followed by acetic acid (0.5%) to irritate the bladder. One hour after starting acetic acid infusion, a protocol consisting of a cystometrogram, continuous infusion-induced rhythmic voiding contractions, and a 5 min "quiet period" (bladder emptied without infusion) was precisely repeated every 30 minutes. Administration of vehicle (saline i.v.) occurred 15 minutes after starting each of the first 7 cystometrograms and duloxetine (1mg/kg i.v.) after the 8(th). Acetic acid infusion into the bladder increased URS-EMG activity, bladder contraction frequency, and decreased contraction amplitude and capacity, compared to saline. Bladder activity and URS activity stabilized within 1 and 2 hours, respectively. Duloxetine administration significantly decreased bladder contraction frequency and increased URS-EMG activity to levels similar to previous reports. Cardiorespiratory parameters and blood gas levels remained consistent throughout the experiment. The epithelium of the bladder and urethra were greatly damaged and edema and infiltration of neutrophils in the lamina propria of urethra were observed. These data provide an ample evaluation of the health of the animals, stability of voiding function and appropriateness of the model for testing drugs designed to evaluate lower urinary tract as well as cardiovascular and respiratory systems function.
Conflict of interest statement
Figures



Similar articles
-
Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat.J Pharmacol Exp Ther. 1995 Aug;274(2):1014-24. J Pharmacol Exp Ther. 1995. PMID: 7636716
-
Comparison of the effects of serotonin selective, norepinephrine selective, and dual serotonin and norepinephrine reuptake inhibitors on lower urinary tract function in cats.Life Sci. 2002 Aug 2;71(11):1227-36. doi: 10.1016/s0024-3205(02)01848-9. Life Sci. 2002. PMID: 12106588
-
alpha-Chloralose alters autonomic reflex function of the lower urinary tract.Am J Physiol. 1991 Dec;261(6 Pt 2):R1560-7. doi: 10.1152/ajpregu.1991.261.6.R1560. Am J Physiol. 1991. PMID: 1750580
-
Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: implications for treating stress urinary incontinence.Urology. 2003 Oct;62(4 Suppl 1):3-9. doi: 10.1016/s0090-4295(03)00754-4. Urology. 2003. PMID: 14550831 Review.
-
Detrusor-sphincter dyssynergia.Vet Clin North Am Small Anim Pract. 1996 Mar;26(2):327-38. doi: 10.1016/s0195-5616(96)50213-5. Vet Clin North Am Small Anim Pract. 1996. PMID: 8711868 Review.
Cited by
-
Neurotransmitter Mechanisms Underlying Sacral Neuromodulation of Bladder Overactivity in Cats.Neuromodulation. 2017 Jan;20(1):81-87. doi: 10.1111/ner.12534. Epub 2016 Oct 12. Neuromodulation. 2017. PMID: 27730701 Free PMC article.
-
Sex difference in the contribution of GABAB receptors to tibial neuromodulation of bladder overactivity in cats.Am J Physiol Regul Integr Comp Physiol. 2017 Mar 1;312(3):R292-R300. doi: 10.1152/ajpregu.00401.2016. Epub 2016 Dec 14. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 27974317 Free PMC article.
-
Stimulation of the pelvic nerve increases bladder capacity in the PGE2 cat model of overactive bladder.Am J Physiol Renal Physiol. 2020 Jun 1;318(6):F1357-F1368. doi: 10.1152/ajprenal.00068.2020. Epub 2020 Apr 20. Am J Physiol Renal Physiol. 2020. PMID: 32308021 Free PMC article.
-
Development and Validation of a Quantitative UHPLC-MS-MS Method for the Determination of Alpha-Chloralose in Feline Blood and Application on Blood Samples Collected from Cats with Symptoms of Alpha-Chloralose Poisoning.J Anal Toxicol. 2022 Jul 14;46(6):651-657. doi: 10.1093/jat/bkab087. J Anal Toxicol. 2022. PMID: 34313718 Free PMC article.
-
Real-time prediction of bladder urine leakage using fuzzy inference system and dual Kalman filtering in cats.Sci Rep. 2024 Feb 16;14(1):3879. doi: 10.1038/s41598-024-53629-5. Sci Rep. 2024. PMID: 38365925 Free PMC article.
References
-
- Fowler CJ, Griffiths D, de Groat WC (2008) The neural control of micturition. Nat Rev Neurosci 9: 453-466. doi:10.1038/nrn2401. PubMed: 18490916. - DOI - PMC - PubMed
-
- de Groat WC (2006) Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147 Suppl 2: S25-S40. doi:10.1038/sj.bjp.0706604. PubMed: 16465182. - DOI - PMC - PubMed
-
- Thor KB, de Groat WC (2010) Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. Am J Physiol Regul Integr Comp Physiol 299: R416-R438. doi:10.1152/ajpregu.00111.2010. PubMed: 20484700. - DOI - PMC - PubMed
-
- Karicheti V, Stone EA, Langdale CL, Katofiasc MA, Brooks JD et al. (2006) Chronic real-time ambulatory urodynamics in cats using radio telemetry for drug screening. 2006 AUA, Annual Meeting. Atlanta, GA.
-
- Stone EA, Karicheti V, Langdale CL, Katofiasc MA, Brooks JD et al. (2005) Long-term, Real-Time ambulatory urodynamics in cats using radio telemetry (TTY). Society for Neuroscience; Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

