Functional characterization of a juvenile hormone esterase related gene in the moth Sesamia nonagrioides through RNA interference
- PMID: 24040087
- PMCID: PMC3770702
- DOI: 10.1371/journal.pone.0073834
Functional characterization of a juvenile hormone esterase related gene in the moth Sesamia nonagrioides through RNA interference
Abstract
Juvenile hormone esterase (JHE) is a carboxylesterase that has attracted great interest because of its critical role in regulating larval to adult transition in insects and other arthropods. Previously, we characterized an ecdysteroid sensitive and juvenile hormone non-susceptible juvenile hormone esterase related gene (SnJHER) in the corn stalk borer, Sesamia nonagrioides. SnJHER was rhythmically up-regulated close to each molt during the corn stalk borer's larval development. In this paper we attempted to functionally characterize SnJHER using several reverse genetics techniques. To functionally characterize SnJHER, we experimented with different dsRNA administration methods, including hemolymph, bacterial or baculovirus-mediated RNA interference, (RNAi). Our findings indicate the potential implication of SnJHER in the developmental programming of Sesamia nonagrioides. It is still unclear whether SnJHER is closely related to the authentic JHE gene, with different or similar biological functions.
Conflict of interest statement
Figures

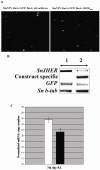



Similar articles
-
Molecular characterization of an ecdysteroid inducible carboxylesterase with GQSCG motif in the corn borer, Sesamia nonagrioides.J Insect Physiol. 2011 Jul;57(7):1000-9. doi: 10.1016/j.jinsphys.2011.04.017. Epub 2011 Apr 27. J Insect Physiol. 2011. PMID: 21549123
-
Juvenile hormone esterase activity in the pupating and diapausing larvae of Sesamia nonagrioides.J Insect Physiol. 2008 Jun;54(6):916-21. doi: 10.1016/j.jinsphys.2008.04.015. Epub 2008 Apr 30. J Insect Physiol. 2008. PMID: 18519138
-
A whole genome screening and RNA interference identify a juvenile hormone esterase-like gene of the diamondback moth, Plutella xylostella.J Insect Physiol. 2015 Sep;80:81-7. doi: 10.1016/j.jinsphys.2015.02.001. Epub 2015 Feb 24. J Insect Physiol. 2015. PMID: 25721055
-
Identification and characterization of juvenile hormone esterase gene from the yellow fever mosquito, Aedes aegypti.Insect Biochem Mol Biol. 2007 Aug;37(8):829-37. doi: 10.1016/j.ibmb.2007.05.010. Epub 2007 May 29. Insect Biochem Mol Biol. 2007. PMID: 17628281 Free PMC article.
-
Insecticidal properties of genetically engineered baculoviruses expressing an insect juvenile hormone esterase gene.Appl Environ Microbiol. 1992 May;58(5):1583-91. doi: 10.1128/aem.58.5.1583-1591.1992. Appl Environ Microbiol. 1992. PMID: 1622228 Free PMC article.
Cited by
-
Transcriptome-Based Identification of a Functional Fasciola hepatica Carboxylesterase B.Pathogens. 2021 Nov 10;10(11):1454. doi: 10.3390/pathogens10111454. Pathogens. 2021. PMID: 34832612 Free PMC article.
-
Comparative transcriptome reveals the potential modulation mechanisms of estradiol affecting ovarian development of female Portunus trituberculatus.PLoS One. 2019 Dec 19;14(12):e0226698. doi: 10.1371/journal.pone.0226698. eCollection 2019. PLoS One. 2019. PMID: 31856263 Free PMC article.
-
The molecular and physiological impact of bisphenol A in Sesamia nonagrioides (Lepidoptera: Noctuidae).Ecotoxicology. 2015 Mar;24(2):356-67. doi: 10.1007/s10646-014-1384-6. Epub 2014 Dec 10. Ecotoxicology. 2015. PMID: 25492584
-
Transcriptional response of immune-related genes after endogenous expression of VP1 and exogenous exposure to VP1-based VLPs and CPV virions in lepidopteran cell lines.Mol Genet Genomics. 2019 Aug;294(4):887-899. doi: 10.1007/s00438-019-01551-1. Epub 2019 Mar 28. Mol Genet Genomics. 2019. PMID: 30923941
-
Insecticidal Gene Silencing by RNAi in the Neotropical Region.Neotrop Entomol. 2020 Feb;49(1):1-11. doi: 10.1007/s13744-019-00722-4. Epub 2019 Nov 20. Neotrop Entomol. 2020. PMID: 31749122 Review.
References
-
- Ranson H, Claudianos C, Ortelli F, Abgrall C, Heminway J, et al. (2002) Evolution of supergene families associated with insecticide resistance. Science 298: 179–181. - PubMed
-
- Teese MG, Campbell PM, Scott C, Gordon KH, Southon A, et al. (2010) Gene identification and proteomic analysis of the esterases of the cotton bollworm, Helicoverpa armigera . Insect Biochem Mol Biol 40: 1–16. - PubMed
-
- Tsubota T, Shimomura M, Ogura T, Seino A, Nakakura T, et al. (2010) Molecular characterization and functional analysis of novel carboxyl/cholinesterases with GQSAG motif in the silkworm Bombyx mori . Insect Biochem Mol Biol 40: 100–12. - PubMed
-
- Hammock BD (1985) Regulation of juvenile hormone titer: degradation. In: Kerkut, G.A, Gilbert, L.I., Editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. New York: Pergamon Press. 431–472.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

